The obstructive sleep apnea syndrome (OSA) is a clinical entity characterized by recurring episodes of apnea and/or hypopnea during sleep, due to a total or partial collapse, respectively, of the upper airway. This collapse originates a set of pathophysiological changes that determine the appearance of several cardiovascular complications. OSA contributes for the development of hypertension, heart failure, arrhythmias and coronary heart disease. Nowadays it is recognized to be an important public health problem, taking into account not just its repercussions but also its prevalence, since the main risk factor for the disease is obesity, a growing problem worldwide, both in developed and developing countries. The present review summarizes the current knowledge about OSA, as regards its definition, pathophysiology, clinical manifestations, diagnosis, cardiovascular effects and treatment.
El síndrome de apnea-hipopnea del sueño (SAHS) es una entidad clínica caracterizada por episodios de apnea y/o hipopnea recurrentes durante el sueño, debido a un colapso total o parcial, respectivamente, de la vía aérea superior. Este colapso origina un conjunto de cambios fisiopatológicos que determinan la aparición de diversas complicaciones cardiovasculares, contribuyendo al desarrollo de hipertensión arterial, insuficiencia cardiaca, arritmias y enfermedad arterial coronaria. Hoy en día se reconoce como un importante problema de salud pública, teniendo en cuenta no sólo sus consecuencias, sino también su elevada prevalencia. Uno de los principales factores de riesgo del SAHS es la obesidad, un problema de gran relevancia en los países desarrollados y en vías de desarrollo. La presente revisión resume el conocimiento actual sobre la AOS, en cuanto a su definición, fisiopatología, manifestaciones clínicas, diagnóstico, efectos cardiovasculares y tratamiento.
The obstructive sleep apnea syndrome (OSA) is a clinical entity characterized by recurring episodes of apnea and/or hypopnea during sleep, occurring by total or partial collapse, respectively, of the upper airway, in a frequency superior to five times per hour of sleep.1
This collapse originates a set of pathophysiological changes that determine the appearance of several cardiovascular complications. OSA can be an independent cause of systemic arterial and pulmonary hypertension; it can also contribute for the development of heart failure, arrhythmias and coronary artery disease.
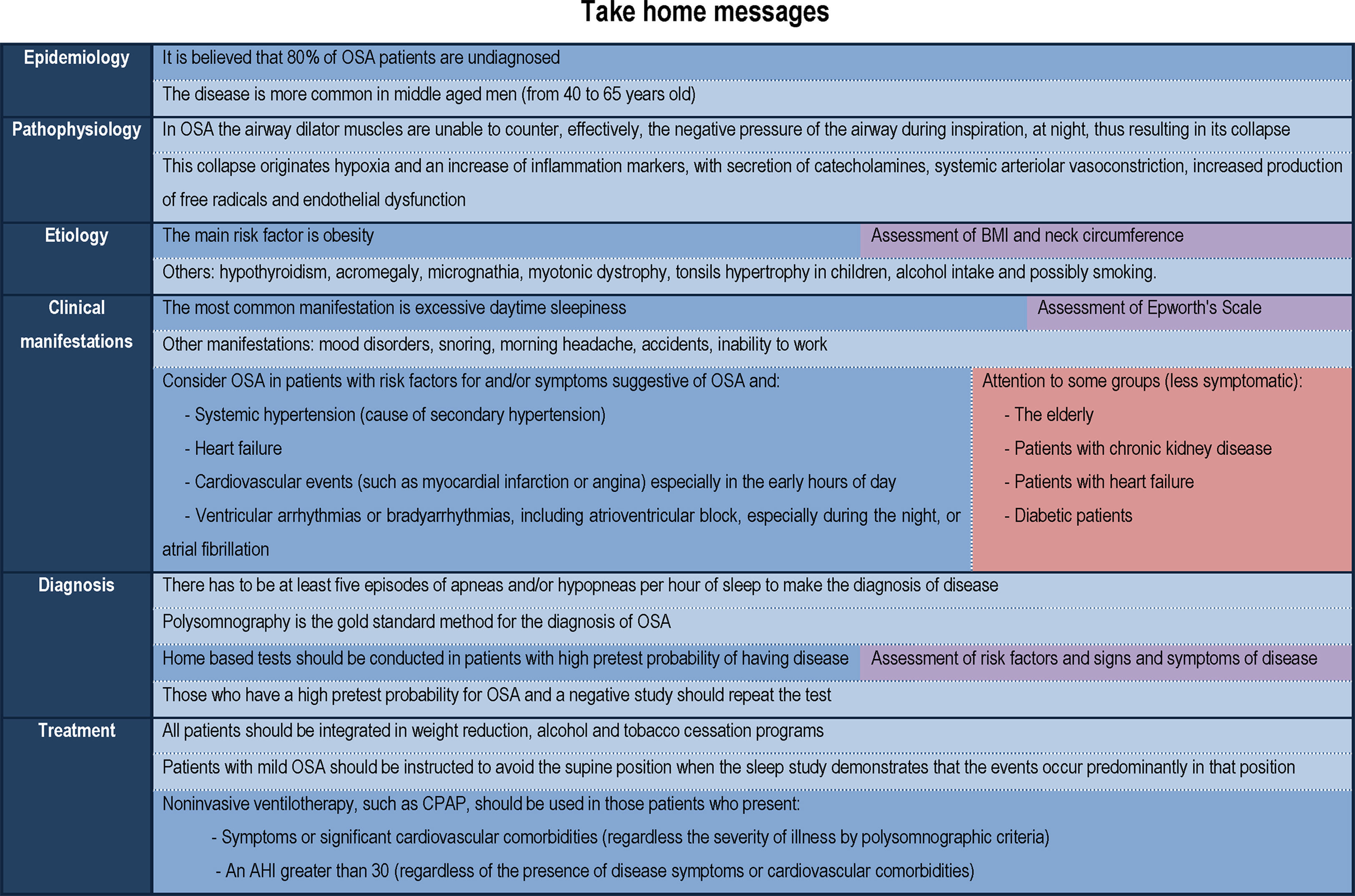
Nowadays it is recognized to be an important public health problem, taking into account its prevalence, severity and socio-economic impact.2 It is a problem that has been underestimated and it is believed that 80% of OSA patients are undiagnosed.1,2 Therefore, the precocious recognition of this disease and institution of interventional measures are mandatory.
The present review summarizes the current knowledge about OSA, as regards its pathophysiology, clinical manifestations, diagnosis, cardiovascular repercussions and treatment.
EpidemiologyOSA is an important public health problem, whose knowledge has grown over the last five decades.1 It has been underestimated, and it is believed that 80% of OSA patients are undiagnosed.1,2 Nowadays it is recognized to be a preponderant cardiovascular risk factor, since its main cause is obesity, a growing problem worldwide, both in developed and developing countries.1
It is estimated to affect approximately 9% of females and 24% of males, considering the adult population in general.3 The disease is more common in middle aged men (from 40 to 65 years old).1
PathophysiologyOSA is characterized by recurring episodes of apnea and/or hypopnea during sleep, due to total or partial collapse, respectively, of the upper airway, in a frequency superior to five times per hour of sleep.1 Apnea is defined as a reduction of at least 80% of the ventilatory flow; hypopnea corresponds to a reduction of at least 20% of the ventilator flow.4 Both events have to last at least 10s.4
OSA is based on the fact that during the night the airway dilator muscles are unable to counter, effectively, the negative pressure of the airway during inspiration, thus resulting in its collapse.1 During the day, the patency of the airway is maintained at the expense of additional muscular effort, but during sleep the muscle tone decreases, so the airway collapses.
This collapse originates, from a pathophysiological point of view, a nocturnal desaturation followed by reoxygenation (intermittent hypoxemia) which leads to: (1) an activation of carotid chemoreceptors that triggers the secretion of catecholamines and systemic arteriolar vasoconstriction5; (2) an increased production of free radicals5; (3) an increase of inflammation markers, including C-reactive protein and inflammatory cytokines such as TNF-alpha and IL-65,6; and (4) endothelial dysfunction.5 However, there is still no reliable inflammation marker for the diagnosis of disease.6
EtiologyOne of the leading risk factors for OSA is obesity and overweight, which cause narrowing of pharynx,1 a risk factor that is growing around the world, in developing and developed countries. Not just body mass index but also neck circumference seems to be an important risk factor for disease, both being highly correlated.7 Thus, an elevated neck circumference is considered a new clinical parameter that seems to be an independent risk factor for severe OSA.7
Hypothyroidism and acromegaly also predispose to OSA by infiltration of the cervical soft tissues.1 Other risk factors include micrognathia, myotonic dystrophy, Ehlers–Danlos syndrome and possibly smoking.1
Alcohol intake increases the frequency and duration of apneas due to its combined effect of reducing muscle respiratory tone and depression of respiratory center.4
The syndrome can also occur in children usually associated with tonsils hypertrophy.1
Clinical manifestationsThe most common neuropsychiatric manifestation is excessive daytime sleepiness, secondary to sleep fragmentation.8 This drowsiness can cause inability to work effectively, and may affect interpersonal relationships.1 Another consequence is the increased risk of accidents, including traffic and labor accidents. These patients may also present mood disorders, cognitive dysfunction, snoring and morning headache.1
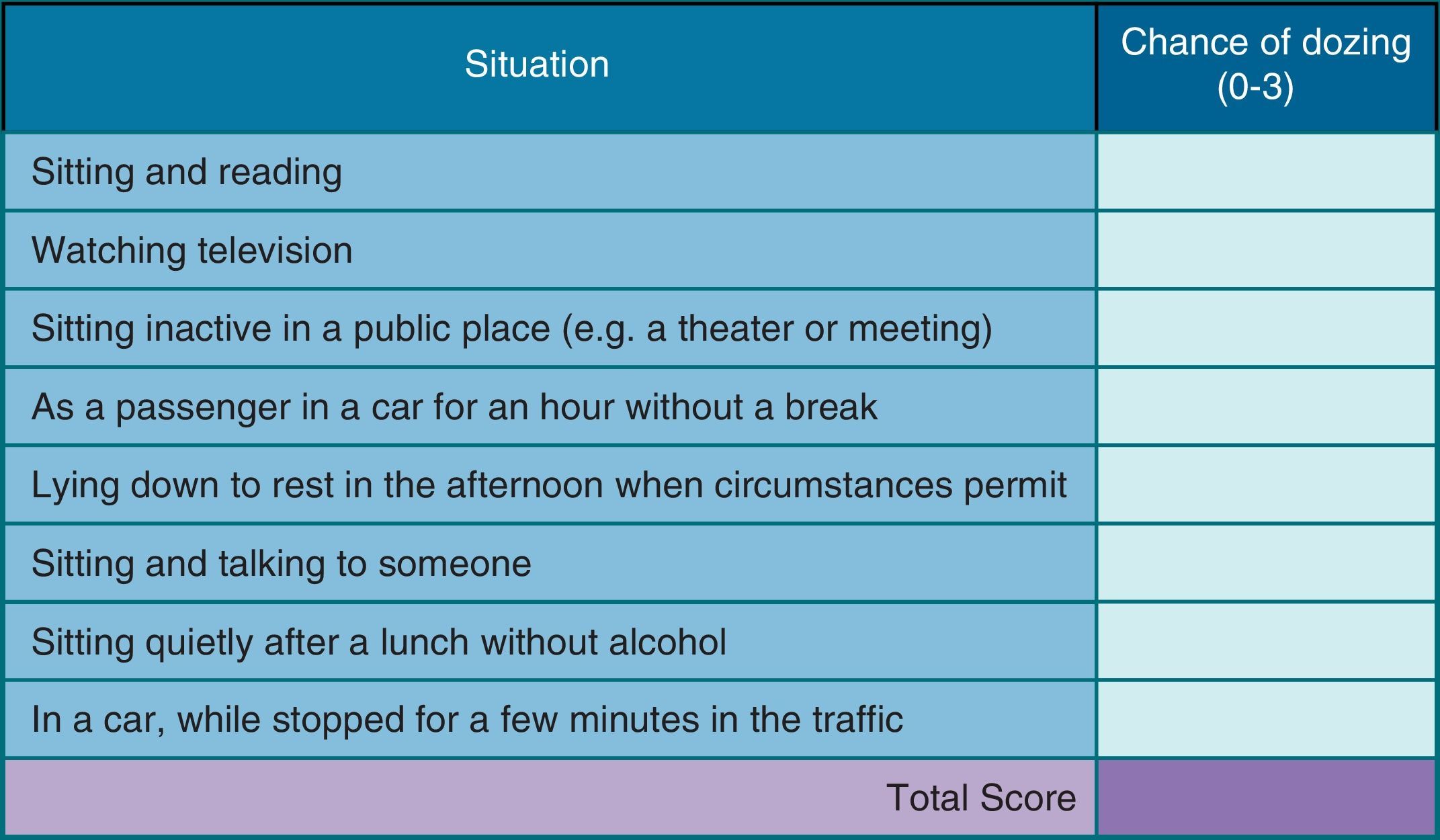
Drowsiness may be assessed by subjective and objective tests.9 Objective tests include Multiple Sleep Latency Testing and Maintenance of Wakefulness Test. However, these tests are expensive, time consuming and difficult to implement in daily clinical practice.9 Subjective tests include Epworth's Scale (Fig. 1), a simple and validated method, first described in 1991, which assesses, through a questionnaire of 8 questions (each with a severity score ranging from 0 to 3, which determines an overall score between 0 and 24), the probability of patient falling asleep in various situations of day.9 It is easy to apply, and is currently the most widely used test for assessment of daytime sleepiness, however, its subjective nature can limit the true perception of the degree of sleepiness prior to treatment.9
Epworth's Scale, which consists of a questionnaire of 8 questions that evaluate the probability of a patient falling asleep in various situations of day. To each question is given a severity score ranging from 0 to 3, which determines an overall score between 0 and 24. A score of 11–15 indicates the possibility of mild to moderate sleep apnea and a score of 16 or above indicates the possibility of severe sleep apnea.
Several authors have described the concept of response shift which is defined as a change of perception over time; it seems that, only after effective treatment of OSA, patients are able to correctly assess the degree of sleepiness prior to the beginning of treatment, with a significant change in Epworth Sleepiness Scale scores between baseline evaluation and the one which is effected retrospectively after initiation of therapy for disease.9
The Epworth Sleepiness Scale may not be the optimal instrument in some particular populations: the elderly, often with atypical presentation of disease10; patients with chronic kidney disease not on dialysis,11 with heart failure12 or diabetes mellitus,13 who seem to have lower prevalence of symptoms such as excessive daytime sleepiness or snoring.
DiagnosisPolysomnography is the gold standard method for the diagnosis of OSA, but is expensive and involves performing an overnight study in a clinical center. Alternative studies carried out at home are a more natural way to assess the sleep.10 These cardiorespiratory studies include the evaluation of various parameters including the respiratory movements, ventilatory flow, heart rate (or electrocardiography) and peripheral oxygen saturation.4
Nowadays it is recommended that the home based tests are conducted in patients with high pretest probability of having disease (which results from the assessment of risk factors and signs and symptoms of disease).4,10 As noted previously, a major problem that arises is that the pretest probability may be different in distinct populations, due to the absence of typical symptoms in some subgroups of patients.10
The sleep study allows the diagnosis and classification of the disease according to the apnea–hypopnea index (AHI), which corresponds to the number of events (apneas and/or hypopneas) per hour of sleep, in mild, moderate or severe form.1 Mild forms have 5–15 events per hour; moderate forms have 15–30 events per hour and severe disease is characterized by more than 30 events per hour.1,4
An important aspect of the evaluation of one-night AHI is that the probability of the observed value is undervalued. The ideal approach to any diagnostic test would be to get several measurements, but in the case of a sleep study, such would be unaffordable because of the costs and inconvenience.14 One study concluded that the supine dominance has a significant impact of incorrectly estimating the AHI.14 Since gravity plays a key role in the positioning of the tongue and soft tissue that occlude the airway, telling the patient to maintain the supine position during the study reduces the underestimation of AHI. However, there is always the question of the real value of the individual. Some portable devices allow assessment of body position. Therefore, those who have a high pretest probability for OSA and a negative study should repeat the test.14
OSA and cardiovascular and cerebrovascular repercussionsIn addition to the social impact and reduced quality of life already mentioned, this disease is associated frequently with cardiovascular and cerebrovascular diseases.
HypertensionIt is estimated that the prevalence of hypertension in OSA patients varies between 35 and 80%.4 On the other hand, 40% of hypertensive patients are diagnosed OSA.4 Thus, if a hypertensive patient has risk factors for and/or symptoms suggestive of OSA, this should be considered as a possible cause of secondary hypertension. OSA is a major risk factor for resistant hypertension.4,8,15 Patients with severe OSA tend to have a non-dipper hypertension and morning hypertension (soon after waking).4,8,15
Hypertension in OSA results from activation of sympathetic nervous system during episodes of apnea–hypopnea, with consequent vasoconstriction.5 In the presence of maintained hypertension there is an increased risk of developing left ventricular hypertrophy8 as well as an increased risk of acute dissection of aorta.16 This last result is explained by the negative intrathoracic pressure generated during the apnea which can change the structure of the aorta as well as the persistent activation of the sympathetic nervous system, which leads to an elevation in blood pressure, culminating in an increased chronic stress in aortic wall.16
Treatment with CPAP (continuous positive pressure ventilation) has shown variable results regarding the benefit in blood pressure control.15,17,18 Several studies observed an improvement in tension profile statistically significant after initiation of non-invasive ventilation; however, this beneficial effect in blood pressure control is variable (more or less modest) and not confirmed by all authors.15,17,18 According to some studies, the benefits may be greater in patients with hypertension refractory to pharmacological therapy.6 A meta-analysis of 12 studies revealed that a greater reduction in blood pressure occurred in younger patients with more severe OSA, with higher frequency of awakenings and greater adherence to treatment.15 The effects of CPAP on ventricular hypertrophy remain controversial for the various authors.19
Heart failureThe actual prevalence of OSA in heart failure patients is unknown since different studies showed variable values. A prospective study evaluating the presence of OSA in a population admitted to a hospital with a diagnosis of heart failure showed the presence of criteria for OSA of moderate to severe degree in 26% of patients.20 In a retrospective analysis of 450 heart failure patients referred to a center for a polysomnography study, the presence of criteria for OSA was verified in 37% of patients.21 In another series of cases which evaluated 81 heart failure patients, criteria for OSA were found in 11% of the population studied.22 The differences of the samples and the polysomnographic criteria used may explain some of these variations between studies. The heart failure patients usually have fewer symptoms such as excessive daytime sleepiness or snoring.12,22 Importantly, these patients have often more complex forms of sleep apnea (like central apnea).
The most direct mechanism by which OSA may lead to left ventricle systolic dysfunction is the elevation of blood pressure. Hypertension is the most common risk factor for the development of left ventricular (LV) hypertrophy and heart failure.23 The production of cytokines, catecholamines, endothelin, and other growth factors (increased in OSA) may also contribute to the development of ventricular hypertrophy, regardless of the presence of hypertension.23
Heart failure can still occur by the presence of negative intrathoracic pressure which triggers an increase in transmural pressure of the left ventricle and, therefore, the afterload, which reduces the relaxation and LV filling capacity. There is, concomitantly, an increase in venous return which causes distention of the right ventricle and changes the movement of the interventricular septum, impairing the filling and diastolic function of LV.5 These changes, together, lead to a decrease in stroke volume and cardiac output. Other mechanisms described for the development of heart failure include: increased sympathetic tone on the heart and vessels, which associated with frequent periods of hypoxemia, has an impact on myocardial contractility; the possible development of coronary artery disease in these patients.23
Treatment with the positive pressure devices allows an increase in intrathoracic pressure, reducing the transmural pressure and, consequently, the afterload, which may lead to an improvement of the ejection fraction of the LV.12 A randomized study involving 24 patients reported an increase in ejection fraction of 9% at 30 days of treatment with CPAP; another randomized study reported more modest increase (about 5%) in ejection fraction after 3 months with CPAP.23 The differences in the methodology of the various studies and characteristics of the patients included seem to explain, at least in part, some of the differences found.23
Pulmonary hypertensionIn a study of 220 patients with OSA and apnea–hypopnea index (AHI) greater than 20, there was a prevalence of pulmonary hypertension of 17%, however, it appeared to be predominantly slight.23 Other smaller studies have reported pulmonary hypertension during the day between 20 and 42% of cases.23
Pulmonary hypertension occurs either directly by pulmonary vasoconstriction triggered by hypoxia and hypercapnia, or indirectly by altering the balance of endothelium-derived factors responsible for vasoconstriction and vasodilatation, which leads to hypertrophy and obstructive proliferation of the intima of distal pulmonary arteries, with consequent increase in pulmonary vascular resistance and impaired pulmonary perfusion.5
The CPAP allows, according to some authors, some reversal of pulmonary hypertension.5 However, the results have not shown to be consistent, requiring larger randomized studies. A randomized study showed that effective CPAP therapy was associated with a reduction in systolic pulmonary artery pressure especially in patients who presented at baseline pulmonary hypertension or left ventricular diastolic dysfunction.23
Coronary artery diseaseThe sleep disorders seem to be twice more common in patients with coronary heart disease, compared to patients who do not have coronary disease.23 A recent prospective study that included 73 patients concluded that the association between an AHI greater than 16 and the age of the individual (over 45 years) constituted an important predictor of subclinical atherosclerosis, with a sensitivity of 87% and a specificity of 70.6%.24
In OSA several factors appear to be potential triggers of ischemia including the intermittent severe hypoxia, the increased blood pressure, the sympathetic vasoconstriction and changes in intrathoracic and transmural cardiac pressures.23 In a long-term basis not only those factors appear to be crucial but also the endothelial dysfunction and systemic inflammation will lead to progressive damage of the coronary arteries.23 Also other factors such as the increase of hematocrit and blood hyperviscosity enhance the tendency for platelet aggregation and, consequently, the risk of thromboembolism and atherosclerosis.5 In OSA, cardiovascular events occur especially in the early hours of day.25 In a recent study, it was found that the AHI was an independent predictor associated with less myocardial recovery and a larger size of necrotic area three months after acute myocardial infarction.26
Some observational studies have reported that OSA treatment reduces the risk of new cardiovascular events; however, this is still an area that needs further randomized studies.23
Arrhythmias (brady and tachyarrhythmias)Cardiac arrhythmias occur in up to 50% of OSA patients, being more frequent in patients with more severe disease and/or more marked hypoxemia.23 With more prolonged apneas and increased hypoxia there is not only an increase of vagal tone as a frequent stimulation of the sympathetic nervous system, but also there occurs an imbalance between the two systems.27
Ventricular arrhythmias (unsustained ventricular tachycardia and ectopic ventricular complexes) become more frequent with significant hypoxemia,5 especially during the nocturnal period.27
According to some studies, there is a frequent coexistence of sleep problems and the presence of atrial fibrillation (AF),27–29 having already been reported as an association between the number of desaturation events and the emergence of the arrhythmia.27,29 The pathogenesis of atrial fibrillation has several bases including: the thin atrial wall may be more vulnerable to transmural forces that occur with changes in intrathoracic pressure, leading over time to an increase in the size of cardiac chambers and the remodeling of the pulmonary ostium veins27,29; the ostium of the pulmonary veins have a high density of vagal and adrenergic nerves.27,29 It is thought that repeated oxyhemoglobin desaturation can activate ion channels sensitive to catecholamines resulting in focal depolarizations that initiate AF.27 Also systemic inflammation and obesity, often present in OSA, seem to potentiate the emergence of arrhythmia.27,29 OSA is also an independent risk factor for a less durable pulmonary vein isolation in patients undergoing ablation treatment.27,29
Also bradyarrhythmias, including atrioventricular block may appear in these patients (often in the absence of conduction tissue disease), so its presence, especially at night, should raise the suspicion of OSA.23,30 In the electrocardiogram a widening of the QRS interval can occur; a study has shown that the apnea–hypopnea index is independently associated with the QRS duration in women, but not in men.31 The QRS duration appears to be an independent predictor of mortality in conditions commonly associated with OSA, such as hypertension and heart failure.31
There is a strong possibility of effectiveness of CPAP treatment in reducing or abolishing many of the mechanisms linking OSA to the AF, but only a few studies reported this benefit.27 An observational study showed that successful cardioversion in a patient with untreated OSA is associated with an 82% risk of recurrence of atrial fibrillation in less than a year, the double of a patient effectively treated with CPAP.23 In the case of bradyarrhythmias occurring during episodes of apnea, CPAP therapy may be effective in their reversal, in the absence of concomitant conduction tissue disease or thyroid dysfunction.23
Stroke, transient ischemic attack (TIA) and cognitive dysfunctionSixty to eighty percent of patients with stroke and TIA have an AHI higher than 10.32 The silent cerebral infarcts, with no symptoms or predominantly involving small vessel disease are common in patients with sleep apnea,32 occurring in 25% of patients with moderate to severe OSA.32 OSA seems to be not only a risk factor for stroke but can also exacerbate the injury caused by stroke.32 Published studies have also reported an association between OSA and dementia in the elderly, the severity of dementia being related to the sleep problem degree.32 OSA seems to accelerate cognitive decline, the onset and severity of dementia, although not all studies have found a relationship with statistical significance between OSA and cognitive function in elderly.32
OSA and metabolic repercussionsDeterioration of renal functionSome studies found in patients with OSA a possible deterioration of renal function, either directly, by the effect of hypoxia, or indirectly by increased blood pressure, inflammatory cytokines and activation of the sympathetic nervous system.11
Increased insulin resistanceSome studies showed in OSA patients an increase of insulin resistance with a higher risk of developing diabetes.5 There was an independent association between OSA and diabetic neuropathy in patients with diabetes mellitus type 2.13 The severity of diabetic neuropathy appears to be consistent with the severity of OSA.13 Some studies have shown beneficial effect of CPAP therapy on HbA1C (after 3–4 months of therapy); however, this has been difficult to replicate consistently.17
HyperuricemiaHyperuricemia occurs in a significant percentage of patients due to periods of hypoxia that are probably a stimulus to increase the turnover of nucleotides, generating purines which are metabolized in uric acid.33,34 One study showed a relationship between gout and sleep disorders (in general), but there was no statistically significant association between gout and OSA.33
TreatmentChanges in lifestyleTreatment of OSA should always involve changes in lifestyle such as weight reduction and smoking and alcohol cessation.
Although excess weight is the predominant risk factor for OSA, the weight percentage loss required for a substantive improvement in AHI or in the repercussions of the disease is discussed.4 In an observational study it was found that a reduction in weight percentage of about 10% was equivalent to a reduction of AHI of about 26%.4
The real need for sedatives at bedtime (in individuals who take it) should also be addressed, since they contribute to the reduction of airway muscle tone.
Adjustment of body position during sleepSome patients may also benefit by adjustment of body position during sleep. Patients with mild OSA should be instructed to avoid the supine position when the polysomnographic study demonstrates that the events occur predominantly in that position.4
VentilotherapyIn addition to general measures, noninvasive ventilotherapy, such as CPAP, should be used in some patients.
CPAP treatment is the primary form of therapy in these patients, especially in the presence of severity criteria, social and/or important cardiovascular effects and in the absence of other more complex forms of sleep apnea or respiratory disorders (whose presence may indicate the need for other forms of non-invasive ventilation). It allows the reduction of cardiovascular morbidity and mortality.8
Thus the main indications for its prescription are:
- -
The presence of symptoms such as excessive daytime sleepiness, or significant cardiovascular comorbidities (before an AHI greater than 5, and regardless of the severity of illness by polysomnographic criteria)35;
- -
An AHI greater than 30 (index compatible with severe disease), regardless of the presence of disease symptoms or cardiovascular comorbidities.35
Unfortunately 30% of OSA patients do not tolerate CPAP or do not perform it because of discomfort with the mask. In patients asymptomatic or minimally symptomatic non-adherence is still greater.32
SurgeryThere are four types of surgery that can benefit OSA patients: (1) bariatric surgery in patients with morbid obesity; (2) tonsillectomy, a procedure highly effective in children; (3) maxillo-mandibular osteotomy surgery in patients with retrognathia; (4) tracheostomy, which is curative but rarely used because of increased morbidity.1
ConclusionsOSA is an important public health issue, with high prevalence but often underestimated. It has several social consequences, and is an important cardiovascular risk factor, hence the importance of its early recognition and diagnosis in clinical practice. Although this association is well defined with the cardiovascular diseases, sleep apnea remains underdiagnosed, as in the general population, in cardiac patients. It is crucial to organize the health system to provide effective care in the continuum of patient experience with sleep disorders, with the development of programs that increase the level of suspicion of OSA in primary and secondary healthcare. Nowadays the diagnosis can be easily made with tests performed at home in patients with high pretest probability of having the disease, and no other diagnosis suspicion. The sleep study allows the diagnosis and classification of the disease according to the AHI in mild, moderate or severe form. The treatment is based predominantly on measures of general character, including lifestyle changes, and noninvasive ventilotherapy, which seems to have strong current evidence of a favorable impact on many repercussions of the disease.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflict of interestThe authors declare no conflict of interest.