It is a fact that coronary artery disease (CAD) is more prevalent in India as compared to western countries. The major risk factors associated with the early CAD are a high prevalence of diabetes mellitus, atherogenic lipid profile, smoking habits, sedentary lifestyle, low socioeconomic condition and high prevalence of obesity. Is this true for restenosis after drug-eluting stent (DES) implantation and factors associated with it? The main objective of the study was to determine the rate of in-stent restenosis (ISR) in patients with DES and risk factors associated with it from our region.
MethodsIt was a single-center, retrospective cohort study in which 550 patients who underwent DES implantation were included. Patient's demographic data, coronary angiography findings, procedural characteristics and development of ISR were noted.
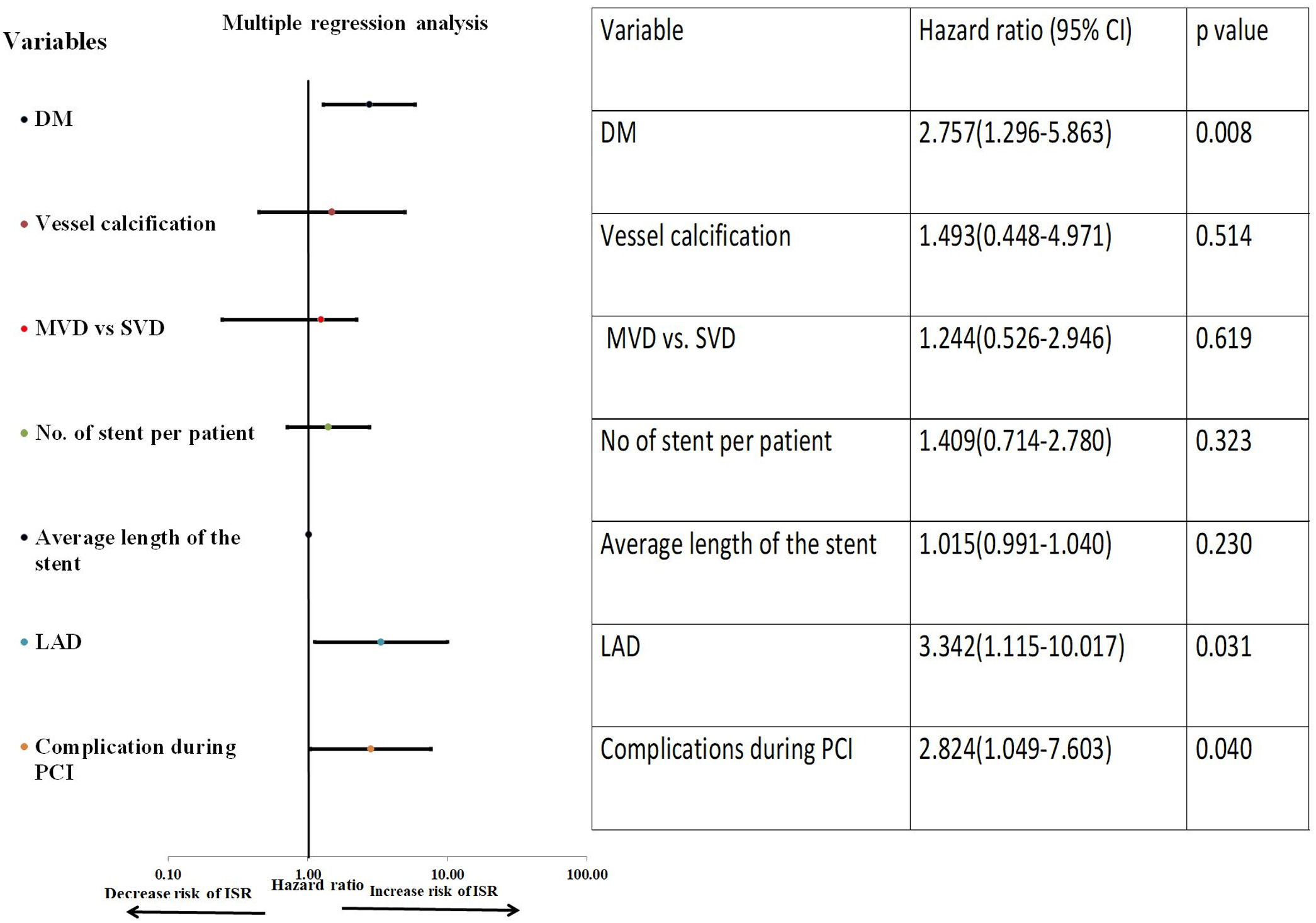
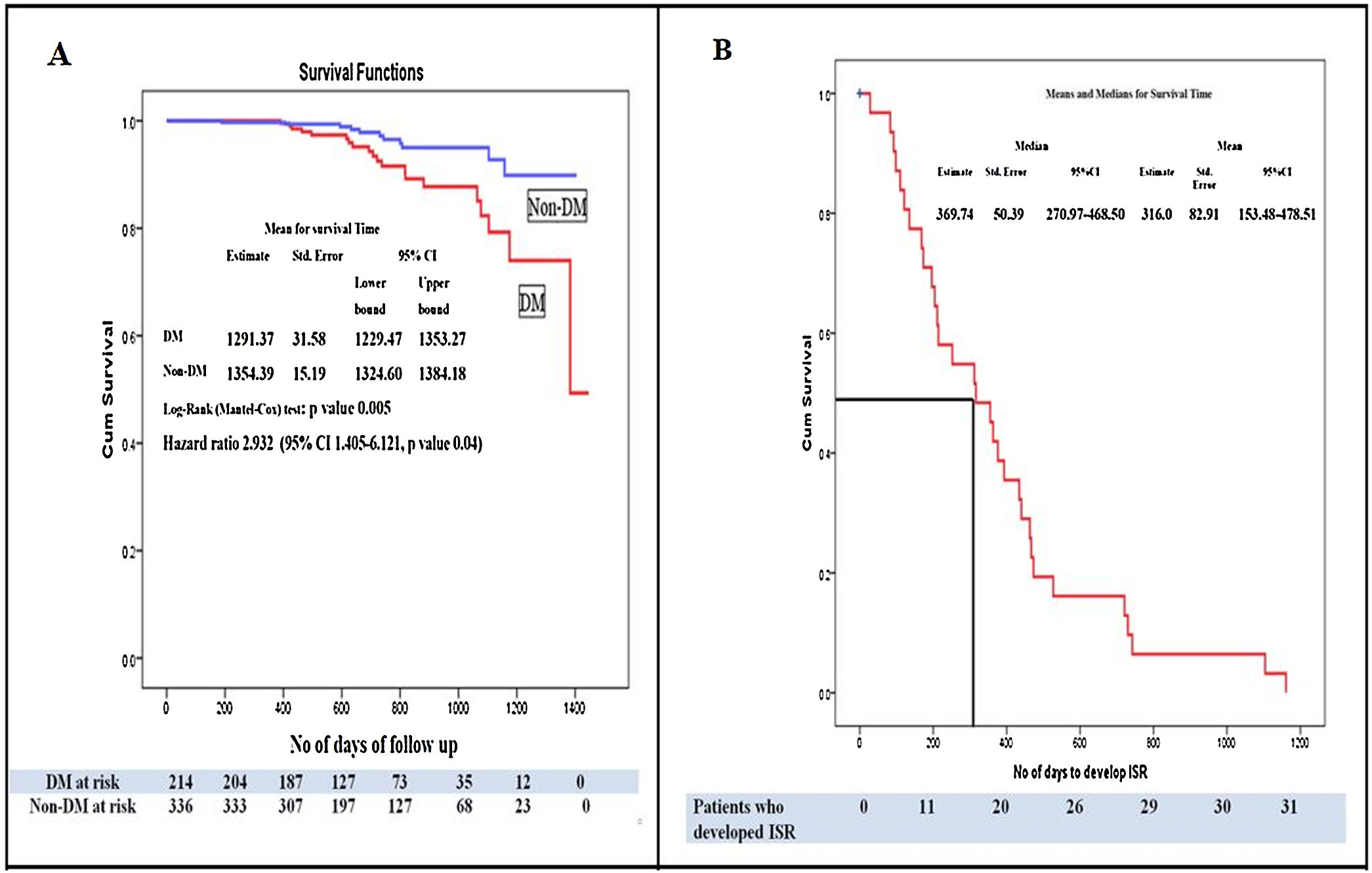
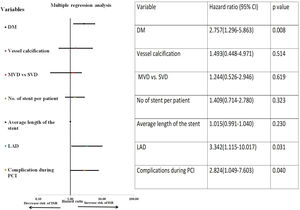
ResultsOut of 550 patients, 31 developed ISR with a rate of restenosis of 5.63% and target lesion revascularization (TLR) of 5.63%. On multiple Cox-regression analysis, only diabetes mellitus (DM) (p=0.008, adjusted hazard ratio (HR): 2.757, 95% confidence interval (CI): 1.296–5.863), deployment of stent in the left anterior descending (LAD) artery (p=0.031, adjusted HR: 3.342, 95% CI: 1.115–10.017) and periprocedural complication during percutaneous coronary intervention (p=0.040, adjusted HR: 2.824, 95% CI: 1.049–7.603) were found to be significantly associated with increased risk of ISR. Kaplan–Meier survival analysis of event-free survival for restenosis showed patients with DM had significantly lower event-free survival compared to patients without DM (p=0.005 by log-rank test).
ConclusionsIn our study, the rate of restenosis after DES implantation was 5.63%. The presence of DM, the stent in the LAD territory and the periprocedural complication is strongly associated with the development of ISR.
Es un hecho que la enfermedad de las arterias coronarias (EAC) es más frecuente en la India que en los países occidentales. Los principales factores de riesgo relacionados con la EAC temprana son una alta prevalencia de la diabetes mellitus, el perfil lipídico aterogénico, el hábito de fumar, el sedentarismo, la baja condición socioeconómica y la alta prevalencia de la obesidad. ¿Es esto cierto para la reestenosis después de la implantación de un stent liberador de fármacos (DES) y los factores asociados a ella? El principal objetivo del El estudio fue determinar la tasa de reestenosis intrastent (ISR) en pacientes con DES y los factores de riesgo asociados a ella de nuestra población.
MétodosFue un estudio de cohorte retrospectivo de un solo centro en el que se incluyeron 550 pacientes que se sometieron a la implantación de DES. Se anotaron los datos demográficos del paciente, los hallazgos de la angiografía coronaria, las características del procedimiento y el desarrollo del ISR.
ResultadosDe 550 pacientes, 31 desarrollaron ISR con una tasa de reestenosis del 5,63% y una revascularización de la lesión diana (TLR) del 5,63%. En el análisis de regresión de Cox múltiple, sólo la diabetes mellitus (p = 0,008, cociente de riesgo ajustado (CRI): 2,757, intervalo de confianza (IC) del 95%: 1,296-5,863), el despliegue de la endoprótesis en la arteria descendente anterior izquierda (LAD) (p = 0,031, CRI ajustado: 3. 342, IC del 95%: 1.115-10.017) y la complicación periprocedimental durante la intervención coronaria percutánea (p = 0.040, CRI ajustado: 2.824, IC del 95%: 1.049-7.603) se encontraron significativamente asociadas con el aumento del riesgo de ISR. El análisis de Kaplan---Meier de supervivencia libre de eventos para la reestenosis mostró que los pacientes con diabetes mellitus (DM) tenían una supervivencia libre de eventos significativamente menor en comparación con los pacientes sin DM (p = 0,005 por prueba de rango logarítmico).
ConclusionesEn nuestro estudio, la tasa de reestenosis tras la implantación del DES fue del 5,63%. La presencia de DM, el stent en el territorio del LAD y la complicación periprocedimental está fuertemente asociada con el desarrollo del ISR.
The management of coronary artery disease (CAD) was significantly changed after the development of balloon angioplasty.1 To reduce the rate of restenosis associated with balloon angioplasty, initially bare-metal stent (BMS) and then drug-eluting stent (DES) was introduced. However, various trials, analyses, meta-analysis and registries had showed that the DES implantation was associated with a significant rate of restenosis depending on the type of lesion treated, and clinical profile of the patient. The rate of restenosis increases with the complexity of the lesion. In simple lesions, the rate of restenosis ranges between 0 and 5%.2,3 However the rates of restenosis in complex lesion range from 5 to 16%.4,5
With the adaptation of DES and a drastic reduction in their cost in developing countries, the number of patients undergoing DES implantation has increased. This has increased the number of patients who are presenting with restenosis. The development of restenosis not only increases the burden on the treating physician but also on the patient. The patient has to undergo multiple interventions that can have a poor psychological impact on the patient. There are various modalities available for the treatment of restenosis.6 However, it was observed that the long term success of restenosis treatment is poorer than the native vessel stenosis treatment.6 Knowledge about the factors associated with restenosis helps the treating physician and patient in making a better decision. There is a lack of large prospective percutaneous coronary intervention (PCI) registries in our country which makes it difficult to predict the rate of restenosis and factors associated with it. It was observed that CAD is more prevalent in India as compared to the western countries. The major risk factors associated with early CAD are a high prevalence of diabetes mellitus (DM), atherogenic lipid profile, smoking habit, sedentary lifestyle, and high prevalence of obesity.7 It was observed that the rate of CAD is three times higher in Asian Indians residing in the USA than the native USA population.8 Can this be true for restenosis after DES implantation and factors associated with it? We still have no answer to this. Various previously conducted studies for restenosis from our region were small in sample size with various methodology flaws and with a loss to follow up.9 All of which makes it difficult to interpret the results of these studies. This study was conducted to determine the rate of in-stent restenosis (ISR) in patients undergoing DES implantation and clinical, angiographic and procedural factors associated with it from a large hospital-based retrospective cohort from our region.
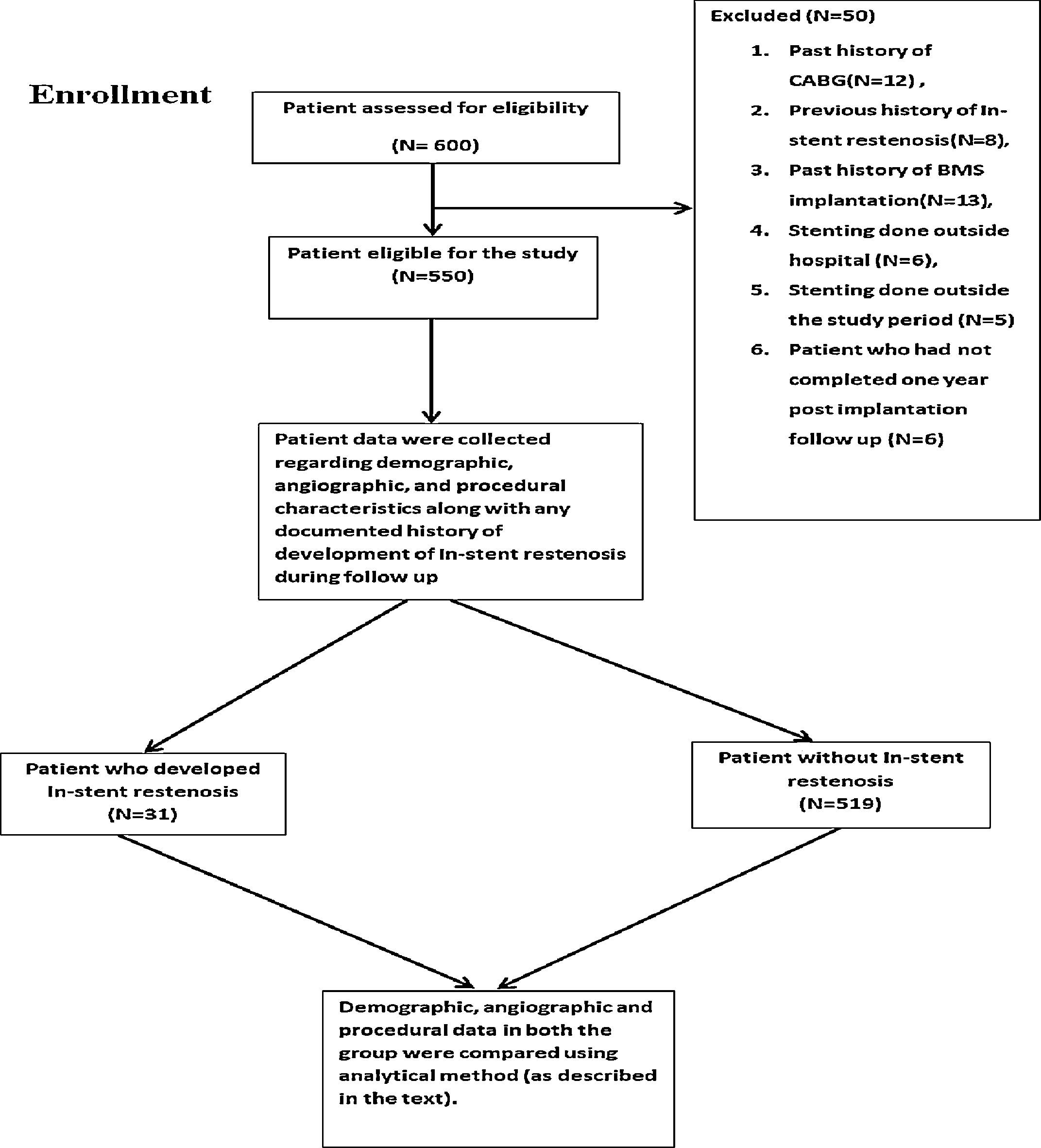
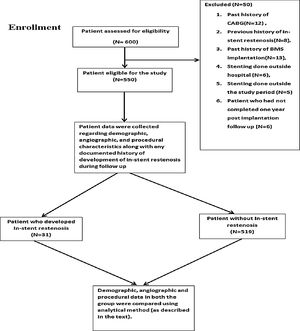
MethodsStudy design and settingIt was a single-center, retrospective cohort study (Supplementary figure) which was conducted in a tertiary care hospital. Institutional ethics committee approval was taken for the study. Written informed consent was taken from the patient. Data collection was started in June 2016 and completed in June 2018.
Participants and follow upIn this study, the patients who underwent DES implantation from 2014 or onward at our center and who had completed at least one-year post-implantation follow up were included. Patients were recruited in the study by individuals who were unaware about the study variable and outcome. All patients underwent PCI according to the standard guidelines and were on maximum medical therapy.
Exclusion criteria for the study include DES implantation outside our institution, history of BMS implantation in the past, stent implantation for the previous ISR, history of coronary artery bypass grafting (CABG), DES implantation before the year 2014, less than one-year post-implantation follow up and patient who died before completing one-year post-implantation follow up period. All patients were under regular follow-up in the cardiology outpatient department (OPD) at one-month interval.
The patient who developed typical symptoms of angina during follow-up after DES implantation underwent repeat coronary angiography (CAG) with or without undergoing stress test (myocardial perfusion imaging or treadmill test) depending on the physician's discretion. The patient who developed acute coronary syndrome (ACS) during follow-up underwent coronary angiogram as per ACC/AHA guidelines. A routine follows up coronary angiogram was not done in the study population for the diagnosis of ISR. Among patients who underwent repeat angiogram, the patient who had ≥50% diameter stenosis of the stented vessel or 5mm proximal or distal to stent margin on visual assessment (done by two independent observers who were blinded to the study) on repeat coronary angiogram were labeled as a case of ISR. The details regarding intervention done in these patients were collected.
Another major event that was collected during the time of data collection was any evidence of stent thrombosis (ST) during the follow-up, compliance regarding the dual antiplatelet therapy in these patients and intervention done for the same. Any cardiac event not related to the stented vessel was not included in the data analysis.
Study objectiveThe primary objective of the study was to determine the rate of ISR in patients with DES. The secondary objective was to determine the clinical, angiographic and procedural characteristics associated with restenosis in patients with DES and time to develop restenosis.
VariableCAD was defined as the presence of binary angiographic diameter stenosis equal to or more than 70%. The multivessel disease was defined as the double vessel or triple vessel CAD. The family history of CAD was defined as the presence of angina, myocardial infarction or revascularization (PCI or CABG) in a first-degree male (age<55 years) or female (<65 years) relative (i.e., parents, siblings, and children) Smoking history was defined as any history of past or present smoking as reported by the patient. History of dyslipidemia was defined as any history of dyslipidemia diagnosed and told in the past by a treating physician, or any documented evidence of dyslipidemia. DM was defined as fasting blood sugar ≥126mg/dl or HbAIC>6.5 or patient who was a diagnosed case of DM and on oral hypoglycaemic agent and or on insulin therapy. Hypertension (HTN) was defined according to JNC-7 guidelines. It was defined as a patient who was a diagnosed case of HTN and on antihypertensive medication or systolic Blood pressure>140mmHg or diastolic BP>90mmHg. Coronary artery calcification was defined as any visible calcification seen on coronary angiogram during any phase of the cardiac cycle.
Coronary thrombus at the time of PCI was defined according to the Academia research consortium (ARC) definition. It was defined as filling defect or lucency which is non-calcified, either spherical or ovoid or irregular in contour which is surrounded by contrast material from the 3 sides or within vessel stenosis and visible in multiple different coronary projection or contrast material persistence within the lumen or downstream visible embolization of intraluminal material.10 Chronic total occlusion (CTO) was defined as total occlusion of the coronary vessel with TIMI 0 flow with duration of occlusion more than 3 months The length of the stent was defined as stent length according to the manufacturer at nominal pressure. In the case of multiple stents in the single vessel, the total of all the stent length was taken as the length of the stent. In a patient who had a stent in multiple vessels, the average length of the stent (sum of all the stent length divided by the number of vessel stented) was taken as stent length. Stent diameter was defined as stent diameter at nominal pressure irrespective of post-dilatation.
Elective PCI was defined as PCI which was done on an outpatient basis or during a hospitalization when the risk of death or myocardial infarction is non-significant. For stable inpatients, elective PCI was defined as when the PCI was performed during the hospitalization according to convenience and easy scheduling and not due to the clinical condition of the patient which needs the procedure to be done before discharge. Primary PCI was defined as PCI performed in patients with ST-segment elevation myocardial infarction (STEMI) within 12h of symptoms onset without any prior fibrinolytic therapy. Left ventricle systolic dysfunction was defined as left ventricular ejection fraction less than 40% as measured by two-dimensional echocardiography. Acute coronary syndrome was defined as a group of syndrome which included patients with STEMI, Non-ST-segment elevation myocardial infarction (NSTEMI) and unstable angina. Myocardial infarction was defined according to a universal definition of STEMI and NSTEMI.
Chronic stable angina (CSA) was defined as typical anginal chest pain which was brought by a predictable level of exertion (or emotional stress) and which remain stable over the last three months. It gets relieved after a short period of rest or with sublingual short-acting nitrates. Periprocedural complications were defined as a complication that occurs during the PCI and was related to the intervention (for example slow flow or no flow leading to myocardial ischemia or complete heart block or an episode of arrhythmias) or instrument (wire, balloon or stent) used during the intervention. The number of days of follow-up was taken as the time from the date of DES implantation to the date of data collection.
Outcome measureISR was defined as binary angiographic diameter stenosis ≥50% at the stent or within 5mm of stent edges in a patient with symptoms of angina or more than 70% in a patient without symptoms. Target lesion revascularization (TLR) was defined as any revascularization in the stented vessel; 5mm proximal and distal to the stent.11 Time to restenosis (or event) was taken as the time from the date of first DES implantation to the date of diagnosis of ISR on coronary angiogram. Event-free survival was defined as survival without any episode of ISR from the date of stent deployment to the date of data collection. ST was defined as per Academic Research Consortium (ARC)-2 definition for ST.11
Data sourceAll the baseline demographic data, hematological parameters, treatment details, angiographic details, and procedural characteristics were collected from the electronic data system of the hospital. The details regarding the first DES implantation was noted from the procedural report available in the electronic data system of the institution. Any data which was not present in the data system was collected from the patient either during follow-up in the OPD or over the telephone. The patient who underwent repeat coronary angiography, detail regarding indication for angiography and result of the angiography was collected from the procedure report available in the hospital records. Details of patients diagnosed as a case of restenosis on angiography and intervention done (PCI, CABG, or medical therapy) in these patients were documented. Data were entered into Microsoft excel 2007 software (Microsoft Corporation, USA).
Study bias and measures taken to reduce themInformation bias or observation bias in the study was reduced by collecting any missing information from the patient either during follow up or over telephonic contact. Selection bias was reduced by the recruitment of patients in the study by the individuals who were not aware of the study protocol. Bias arising due to measurement of a cardiac event (ISR in our study) was reduced by blinding the individual, who was measuring the outcome, for the study protocol. Observer bias was reduced by using standardized questionnaires in all the study patients. Clear and homogenous definition for the variable and outcome were used during data collection. Also, all the data were collected in a similar and uniform method in all the patients.
Data analysisThe categorical variable was expressed in terms of frequency and percentage while the continuous variable was expressed in terms of mean with standard deviation. Association among categorical variable were assessed using chi-square or Fischer-exact test. A comparison of the continuous variable to restenosis status was carried out by using an independent Student t-test (two-sided test). Because of the variable follow up of the patients, the independent factor associated with restenosis was carried out by using univariate and multiple Cox-regression analysis instead of logistic regression analysis. The inclusion criterion for the study was a patient who has at least one-year post-DES implantation follow-up. Patients were recruited at different points of time after DES implantation. This leads to a variable time of follow up in each patient. Given the variable follow up, the cox-proportional hazard model was used instead of logistic regression analysis. Survival function for restenosis was compared using the log-rank test. All the data were analyzed using the SPSS Statistics version 19.0 (IBM Corp., Armonk, NY, USA).
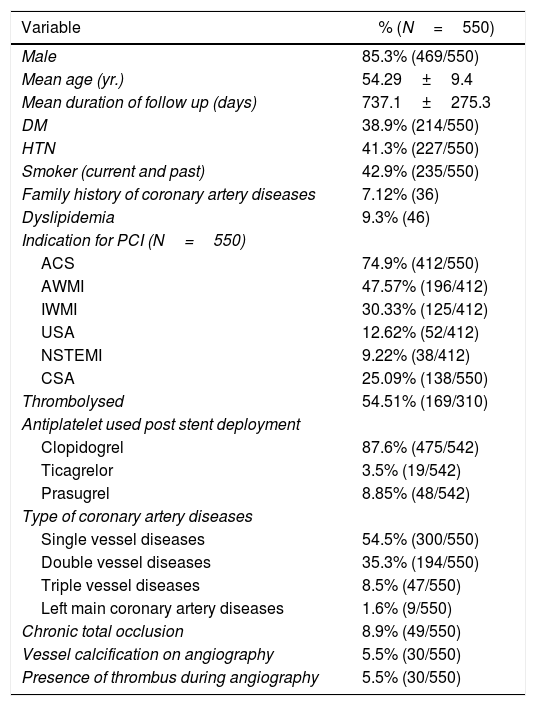
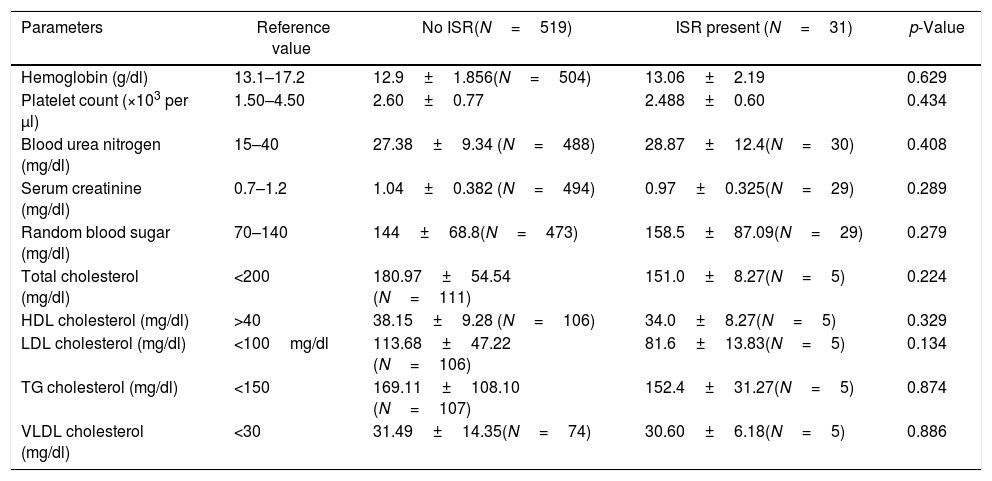
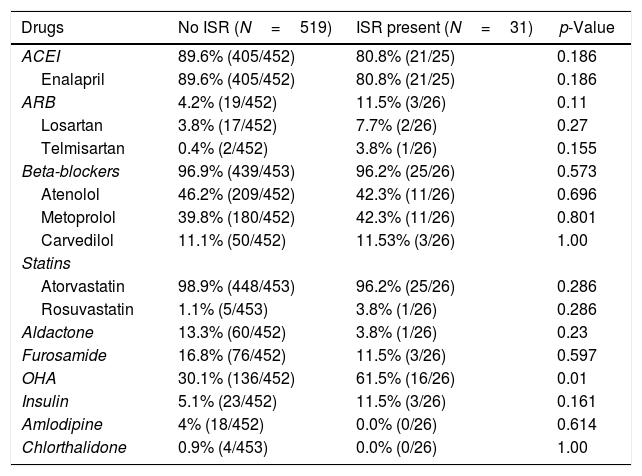
ResultsSix hundred patients were evaluated for the inclusion criteria. Among these, 550 patients were included in the study. Study patients had Paclitaxel/Sirolimus/Everolimus stents. Clinical and angiographic characteristics of the study population are presented in the table (Table 1). There was no significant difference between the two groups (No ISR vs. ISR present) about any laboratory parameters (Table 2). However, significantly more patients in the ISR groups were on oral hypoglycaemic agents as compared to patients with no ISR (p=0.01) (Table 3). The procedural characteristics of the study population are given in the table (Table 4).
Clinical and angiographic characteristics of the study population.
| Variable | % (N=550) |
|---|---|
| Male | 85.3% (469/550) |
| Mean age (yr.) | 54.29±9.4 |
| Mean duration of follow up (days) | 737.1±275.3 |
| DM | 38.9% (214/550) |
| HTN | 41.3% (227/550) |
| Smoker (current and past) | 42.9% (235/550) |
| Family history of coronary artery diseases | 7.12% (36) |
| Dyslipidemia | 9.3% (46) |
| Indication for PCI (N=550) | |
| ACS | 74.9% (412/550) |
| AWMI | 47.57% (196/412) |
| IWMI | 30.33% (125/412) |
| USA | 12.62% (52/412) |
| NSTEMI | 9.22% (38/412) |
| CSA | 25.09% (138/550) |
| Thrombolysed | 54.51% (169/310) |
| Antiplatelet used post stent deployment | |
| Clopidogrel | 87.6% (475/542) |
| Ticagrelor | 3.5% (19/542) |
| Prasugrel | 8.85% (48/542) |
| Type of coronary artery diseases | |
| Single vessel diseases | 54.5% (300/550) |
| Double vessel diseases | 35.3% (194/550) |
| Triple vessel diseases | 8.5% (47/550) |
| Left main coronary artery diseases | 1.6% (9/550) |
| Chronic total occlusion | 8.9% (49/550) |
| Vessel calcification on angiography | 5.5% (30/550) |
| Presence of thrombus during angiography | 5.5% (30/550) |
Data represent mean±SD for continuous variables and % (n) for dichotomous variables.
Abbreviations: ACS: acute coronary syndrome; AWMI: anterior wall myocardial infarction; CSA: chronic stable angina; DM: diabetes mellitus; HTN: hypertension; IWMI: inferior wall myocardial infarction; LWMI: lateral wall myocardial infarction; NSTEMI: non-ST-segment elevation myocardial infarction; USA: unstable angina.
Laboratory profile of patients at the time of stenting.
| Parameters | Reference value | No ISR(N=519) | ISR present (N=31) | p-Value |
|---|---|---|---|---|
| Hemoglobin (g/dl) | 13.1–17.2 | 12.9±1.856(N=504) | 13.06±2.19 | 0.629 |
| Platelet count (×103 per μl) | 1.50–4.50 | 2.60±0.77 | 2.488±0.60 | 0.434 |
| Blood urea nitrogen (mg/dl) | 15–40 | 27.38±9.34 (N=488) | 28.87±12.4(N=30) | 0.408 |
| Serum creatinine (mg/dl) | 0.7–1.2 | 1.04±0.382 (N=494) | 0.97±0.325(N=29) | 0.289 |
| Random blood sugar (mg/dl) | 70–140 | 144±68.8(N=473) | 158.5±87.09(N=29) | 0.279 |
| Total cholesterol (mg/dl) | <200 | 180.97±54.54 (N=111) | 151.0±8.27(N=5) | 0.224 |
| HDL cholesterol (mg/dl) | >40 | 38.15±9.28 (N=106) | 34.0±8.27(N=5) | 0.329 |
| LDL cholesterol (mg/dl) | <100mg/dl | 113.68±47.22 (N=106) | 81.6±13.83(N=5) | 0.134 |
| TG cholesterol (mg/dl) | <150 | 169.11±108.10 (N=107) | 152.4±31.27(N=5) | 0.874 |
| VLDL cholesterol (mg/dl) | <30 | 31.49±14.35(N=74) | 30.60±6.18(N=5) | 0.886 |
Data represent mean±SD for continuous variables.
Abbreviations: HDL: high density lipoprotein; LDL: low density lipoprotein; TG: triglycerides; VLDL: very low-density lipoprotein.
To convert the value of hemoglobin from g/dl to millimoles per liter, multiply by 0.6206. To convert the values for urea nitrogen from mg/dl to millimoles per liter, multiply by 0.357. To convert the values for creatinine from mg/dl to micromoles per liter, multiply by 88.4. To convert the values for glucose to millimoles per liter, multiply by 0.05551. To convert the value of cholesterol (total, HDL-C, LDL-C) from mg/dl to millimoles per liter multiply by 0.0259 and to convert the value of triglyceride from mg/dl to millimoles per liter multiply by 0.0113. The reference range is as recommended by Jawaharlal Institute of Postgraduate Medical Education & Research (JIPMER) Pondicherry (India) for adult population. It may vary according to the hospital, place and age of the patient.
Treatment received by the patient at the time of stenting.
| Drugs | No ISR (N=519) | ISR present (N=31) | p-Value |
|---|---|---|---|
| ACEI | 89.6% (405/452) | 80.8% (21/25) | 0.186 |
| Enalapril | 89.6% (405/452) | 80.8% (21/25) | 0.186 |
| ARB | 4.2% (19/452) | 11.5% (3/26) | 0.11 |
| Losartan | 3.8% (17/452) | 7.7% (2/26) | 0.27 |
| Telmisartan | 0.4% (2/452) | 3.8% (1/26) | 0.155 |
| Beta-blockers | 96.9% (439/453) | 96.2% (25/26) | 0.573 |
| Atenolol | 46.2% (209/452) | 42.3% (11/26) | 0.696 |
| Metoprolol | 39.8% (180/452) | 42.3% (11/26) | 0.801 |
| Carvedilol | 11.1% (50/452) | 11.53% (3/26) | 1.00 |
| Statins | |||
| Atorvastatin | 98.9% (448/453) | 96.2% (25/26) | 0.286 |
| Rosuvastatin | 1.1% (5/453) | 3.8% (1/26) | 0.286 |
| Aldactone | 13.3% (60/452) | 3.8% (1/26) | 0.23 |
| Furosamide | 16.8% (76/452) | 11.5% (3/26) | 0.597 |
| OHA | 30.1% (136/452) | 61.5% (16/26) | 0.01 |
| Insulin | 5.1% (23/452) | 11.5% (3/26) | 0.161 |
| Amlodipine | 4% (18/452) | 0.0% (0/26) | 0.614 |
| Chlorthalidone | 0.9% (4/453) | 0.0% (0/26) | 1.00 |
Data represent % (n) for dichotomous variables.
ACEI: angiotensin converting enzyme inhibitor; ARB: angiotensin receptor blockers; OHA: oral hypoglycaemic agents.
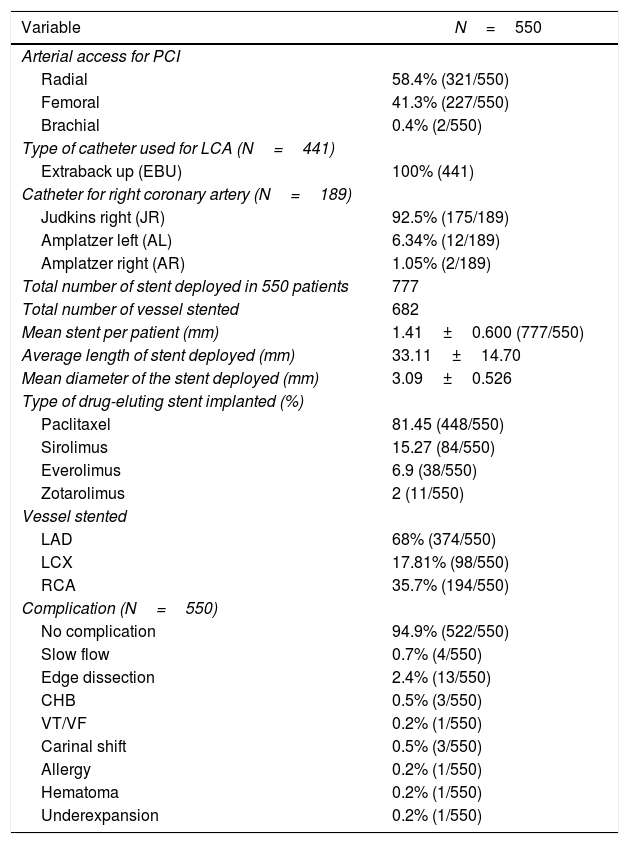
Procedural characteristics of the study population.
| Variable | N=550 |
|---|---|
| Arterial access for PCI | |
| Radial | 58.4% (321/550) |
| Femoral | 41.3% (227/550) |
| Brachial | 0.4% (2/550) |
| Type of catheter used for LCA (N=441) | |
| Extraback up (EBU) | 100% (441) |
| Catheter for right coronary artery (N=189) | |
| Judkins right (JR) | 92.5% (175/189) |
| Amplatzer left (AL) | 6.34% (12/189) |
| Amplatzer right (AR) | 1.05% (2/189) |
| Total number of stent deployed in 550 patients | 777 |
| Total number of vessel stented | 682 |
| Mean stent per patient (mm) | 1.41±0.600 (777/550) |
| Average length of stent deployed (mm) | 33.11±14.70 |
| Mean diameter of the stent deployed (mm) | 3.09±0.526 |
| Type of drug-eluting stent implanted (%) | |
| Paclitaxel | 81.45 (448/550) |
| Sirolimus | 15.27 (84/550) |
| Everolimus | 6.9 (38/550) |
| Zotarolimus | 2 (11/550) |
| Vessel stented | |
| LAD | 68% (374/550) |
| LCX | 17.81% (98/550) |
| RCA | 35.7% (194/550) |
| Complication (N=550) | |
| No complication | 94.9% (522/550) |
| Slow flow | 0.7% (4/550) |
| Edge dissection | 2.4% (13/550) |
| CHB | 0.5% (3/550) |
| VT/VF | 0.2% (1/550) |
| Carinal shift | 0.5% (3/550) |
| Allergy | 0.2% (1/550) |
| Hematoma | 0.2% (1/550) |
| Underexpansion | 0.2% (1/550) |
Data represent mean±SD for continuous variables and % (n) for dichotomous variables.
Abbreviations: CHB: complete heart block; LCA: left coronary artery; VT/VF: ventricular tachycardia/ventricular fibrillation; LAD: left anterior descending; LCX: left circumflex; RCA: right coronary artery.
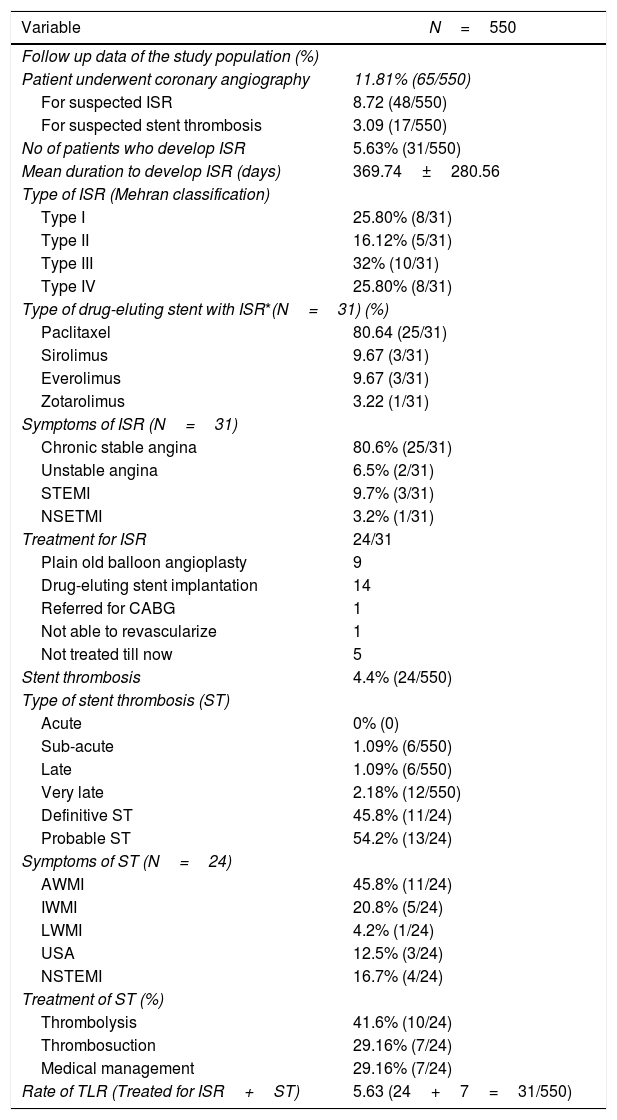
On retrospective data analysis, 31 patients out of 550 patients (5.63%) developed ISR. Out of 550, only 48 patients underwent CAG because of clinical symptoms and or treadmill test or stress thallium suggestive of in-stent restenosis. Out of these 48 patients, only 31 patients were found to have significant ISR. The TLR in the study population was 5.63% (Table 5).
Follow up data of the study population.
| Variable | N=550 |
|---|---|
| Follow up data of the study population (%) | |
| Patient underwent coronary angiography | 11.81% (65/550) |
| For suspected ISR | 8.72 (48/550) |
| For suspected stent thrombosis | 3.09 (17/550) |
| No of patients who develop ISR | 5.63% (31/550) |
| Mean duration to develop ISR (days) | 369.74±280.56 |
| Type of ISR (Mehran classification) | |
| Type I | 25.80% (8/31) |
| Type II | 16.12% (5/31) |
| Type III | 32% (10/31) |
| Type IV | 25.80% (8/31) |
| Type of drug-eluting stent with ISR*(N=31) (%) | |
| Paclitaxel | 80.64 (25/31) |
| Sirolimus | 9.67 (3/31) |
| Everolimus | 9.67 (3/31) |
| Zotarolimus | 3.22 (1/31) |
| Symptoms of ISR (N=31) | |
| Chronic stable angina | 80.6% (25/31) |
| Unstable angina | 6.5% (2/31) |
| STEMI | 9.7% (3/31) |
| NSETMI | 3.2% (1/31) |
| Treatment for ISR | 24/31 |
| Plain old balloon angioplasty | 9 |
| Drug-eluting stent implantation | 14 |
| Referred for CABG | 1 |
| Not able to revascularize | 1 |
| Not treated till now | 5 |
| Stent thrombosis | 4.4% (24/550) |
| Type of stent thrombosis (ST) | |
| Acute | 0% (0) |
| Sub-acute | 1.09% (6/550) |
| Late | 1.09% (6/550) |
| Very late | 2.18% (12/550) |
| Definitive ST | 45.8% (11/24) |
| Probable ST | 54.2% (13/24) |
| Symptoms of ST (N=24) | |
| AWMI | 45.8% (11/24) |
| IWMI | 20.8% (5/24) |
| LWMI | 4.2% (1/24) |
| USA | 12.5% (3/24) |
| NSTEMI | 16.7% (4/24) |
| Treatment of ST (%) | |
| Thrombolysis | 41.6% (10/24) |
| Thrombosuction | 29.16% (7/24) |
| Medical management | 29.16% (7/24) |
| Rate of TLR (Treated for ISR+ST) | 5.63 (24+7=31/550) |
Data represent mean±SD for continuous variables and % (n) for dichotomous variables.
Abbreviations: ISR: in-stent restenosis; ST: stent thrombosis; STEMI: ST-segment elevation myocardial infarction; TLR: target lesion revascularization; (kindly see Table 1 for the remaining abbreviation).
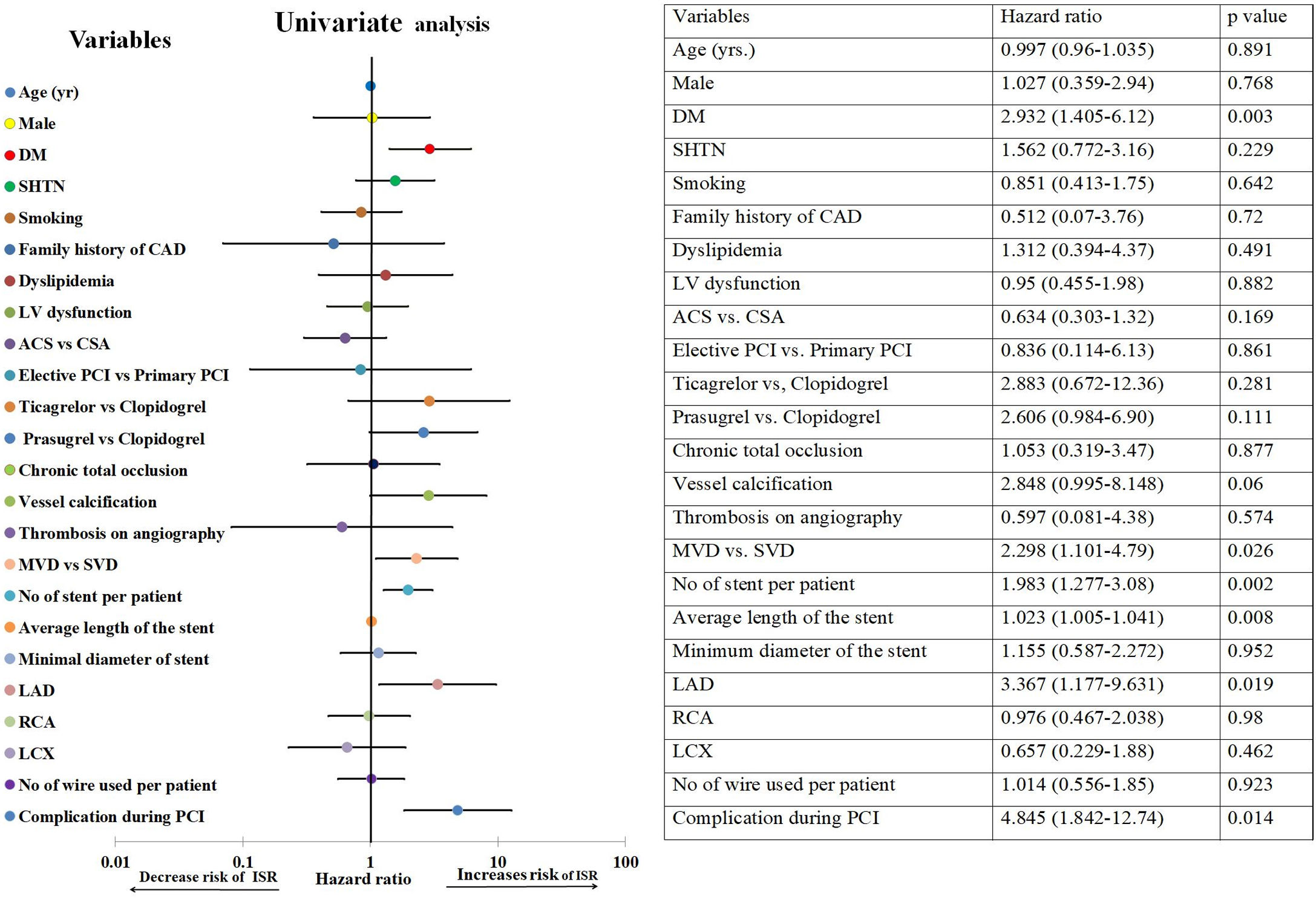
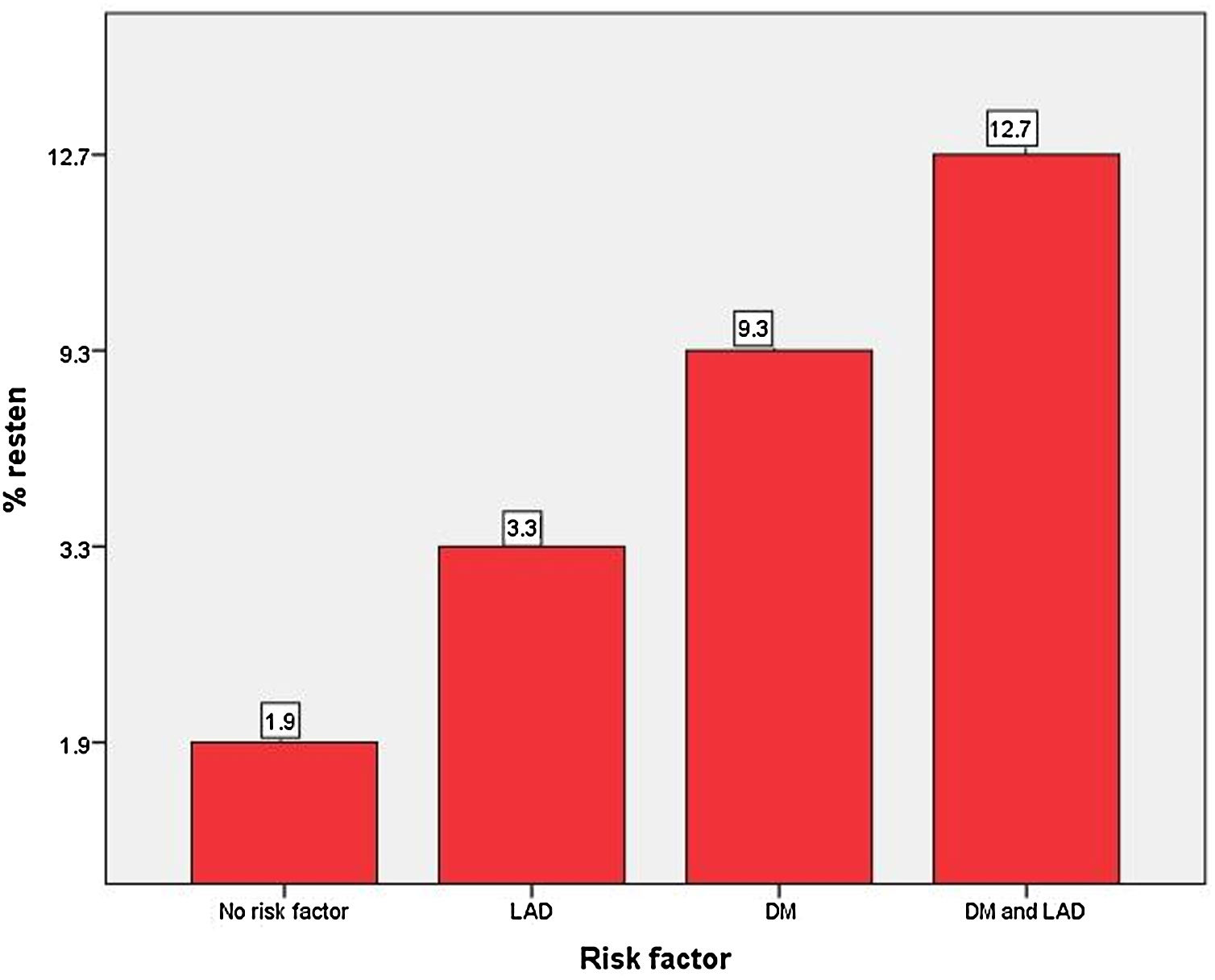
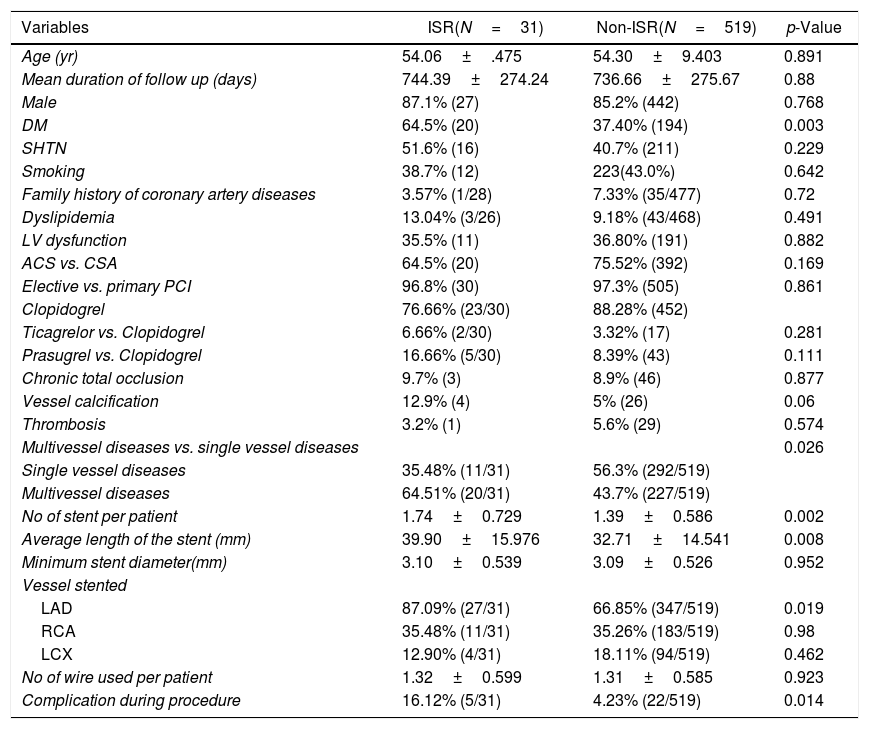
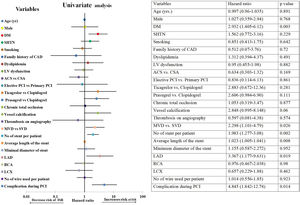
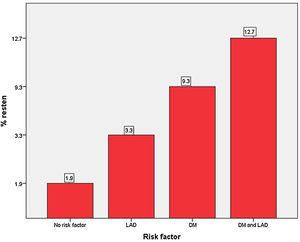
On univariate Cox-regression analysis, DM (p=0.003, odd ratio (OR) 3.046 (1.429–6.493), p=0.003)), presence of calcification (p=0.06), multivessel CAD (p=0.026), the number of stent per patient (p=0.002), an average length of the stent (p=0.008), deployment of the stent in the left anterior descending (LAD) artery (p=0.019, OR: 3.346 (1.152–9.714) (p=0.019)), and periprocedural complication (p=0.014) were associated with increased risk of ISR (Table 6 and Fig. 1). However, on multiple Cox-regression analysis (Fig. 2) only DM (p=0.008, adjusted hazard ratio HR: 2.757, 95% confidence interval CI: 1.296–5.863), deployment of the stent in the LAD (p=0.031, adjusted HR: 3.342, 95% CI: 1.115–10.017), and periprocedural complication during PCI (p=0.040, adjusted HR: 2.824, 95% CI: 1.049–7.603) were found to be significantly associated with increased risk of restenosis. Classification and regression tree analysis showed that the risk of restenosis increases with the increase in the number of risk factors associated with restenosis (Fig. 3).
Univariate analysis of study population for the development of ISR.
| Variables | ISR(N=31) | Non-ISR(N=519) | p-Value |
|---|---|---|---|
| Age (yr) | 54.06±.475 | 54.30±9.403 | 0.891 |
| Mean duration of follow up (days) | 744.39±274.24 | 736.66±275.67 | 0.88 |
| Male | 87.1% (27) | 85.2% (442) | 0.768 |
| DM | 64.5% (20) | 37.40% (194) | 0.003 |
| SHTN | 51.6% (16) | 40.7% (211) | 0.229 |
| Smoking | 38.7% (12) | 223(43.0%) | 0.642 |
| Family history of coronary artery diseases | 3.57% (1/28) | 7.33% (35/477) | 0.72 |
| Dyslipidemia | 13.04% (3/26) | 9.18% (43/468) | 0.491 |
| LV dysfunction | 35.5% (11) | 36.80% (191) | 0.882 |
| ACS vs. CSA | 64.5% (20) | 75.52% (392) | 0.169 |
| Elective vs. primary PCI | 96.8% (30) | 97.3% (505) | 0.861 |
| Clopidogrel | 76.66% (23/30) | 88.28% (452) | |
| Ticagrelor vs. Clopidogrel | 6.66% (2/30) | 3.32% (17) | 0.281 |
| Prasugrel vs. Clopidogrel | 16.66% (5/30) | 8.39% (43) | 0.111 |
| Chronic total occlusion | 9.7% (3) | 8.9% (46) | 0.877 |
| Vessel calcification | 12.9% (4) | 5% (26) | 0.06 |
| Thrombosis | 3.2% (1) | 5.6% (29) | 0.574 |
| Multivessel diseases vs. single vessel diseases | 0.026 | ||
| Single vessel diseases | 35.48% (11/31) | 56.3% (292/519) | |
| Multivessel diseases | 64.51% (20/31) | 43.7% (227/519) | |
| No of stent per patient | 1.74±0.729 | 1.39±0.586 | 0.002 |
| Average length of the stent (mm) | 39.90±15.976 | 32.71±14.541 | 0.008 |
| Minimum stent diameter(mm) | 3.10±0.539 | 3.09±0.526 | 0.952 |
| Vessel stented | |||
| LAD | 87.09% (27/31) | 66.85% (347/519) | 0.019 |
| RCA | 35.48% (11/31) | 35.26% (183/519) | 0.98 |
| LCX | 12.90% (4/31) | 18.11% (94/519) | 0.462 |
| No of wire used per patient | 1.32±0.599 | 1.31±0.585 | 0.923 |
| Complication during procedure | 16.12% (5/31) | 4.23% (22/519) | 0.014 |
Data represent mean±SD for continuous variables and % (n) for dichotomous variables.
Abbreviations: LAD: left anterior descending artery; LCX: left circumflex artery; LV dysfunction: left ventricular systolic dysfunction; RCA: right coronary artery.
Univariate Cox-regression analysis for the development of ISR. There is a significant association of DM, multivessel disease, no. of stent per patient, a total length of the stent, stent in LAD territory and complication during PCI with the development of restenosis. Abbreviations: ACS: acute coronary syndrome; CAD: coronary artery diseases; DM: diabetes mellitus; ISR: in-stent restenosis; LAD: left anterior descending artery; LCX: left circumflex artery; LV dysfunction: left ventricular systolic dysfunction; MVD: multivessel disease; PCI: percutaneous coronary intervention; RCA: right coronary artery; SHTN: hypertension; SVD: single vessel disease.
Multiple Cox-regression analyses for the development of ISR. Among all the factors which were found significant on univariate Cox-regression analysis, only DM, the stent in the LAD territory and periprocedural complication during PCI were significantly associated with the development of ISR. Vessel calcification was also included in multiple analyses as the p-value was 0.051.
Bar diagram for the risk of restenosis. Patients with no risk factor had an ISR rate of 1.9% while a patient with two risk factors had a 12.7% risk for restenosis. Abbreviations: same as in Fig. 1.
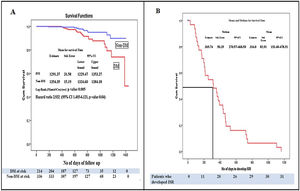
Kaplan–Meier survival analysis of the study population for the time to develop ISR showed that patients with DM developed more ISR as compared to patients without DM (HR: 2.93, 95% CI: 1.40–6.1211, p=0.04). The patients with DM had poor event-free survival as compared to patients without DM, (p=0.005 by log-rank test) (Fig. 4A). Kaplan–Meier survival analysis of patients who developed ISR showed that the median time to develop ISR was 316.0±82.91 days while the meantime to develop ISR was 369.74±50.39 days (Fig. 4B).
(A) Kaplan–Meier survival analysis for the event-free survival of the study population. Patients with diabetes mellitus had significantly lower event-free survival compared to patients without diabetes mellitus (p=0.005 by log-rank test) and hazard ratio of 2.932 (95% CI: 1.405–6.121, p=0.04). (B) Kaplan–Meier curve for the development of ISR. Survival analysis is showing the median time to develop in-stent restenosis. Abbreviations: Non-DM: patient without diabetes mellitus; CI: onfidence interval; ISR: in-stent restenosis; ST: stent thrombosis rests the same as in Figs. 1 and 2.
This study was conducted to find the rate of ISR after DES implantation along with risk factors associated with it. We found that (1) rate of ISR after DES implantation was 5.63%, (2) The rate of TLR in the study population was 5.63%, (3) The development of ISR was strongly associated with the presence of DM, the location of the stent in the LAD territory and periprocedural complication. (4) The median time to develop ISR was 316.0±82.91 days. The mean age of our study population was much less than the mean age of the patients reported in developed countries.12 It has been observed in the previous studies that the CAD occurs 5–10 years earlier in the Indian population than in the western population which is similar to finding in our studies.13
The mean ejection fraction in our study population was 37% which is another poor prognostic marker for the long-term prognosis. Fournier et al. has observed that both functional status and long-term prognosis is poor in a patient who develops CAD at age less than 40 years along with left ventricular systolic dysfunction.14
In our study, PCI had a good procedural success rate in the overall study population. The majority of the studies regarding PCI in young patients observed good short term results. However, on long-term follow-up PCI was found to be associated with the need for repeat revascularization and there was no improvement in overall survival of the patient. The long term prognosis in young patients who underwent PCI depends on the severity of left ventricular systolic dysfunction, prior myocardial infarction, diabetes mellitus, hypertension, tobacco abuse, and active smoking. For this reason, PCI is still considered by many as a palliative procedure in young CAD patients.15 Recently in a study, it was found that CABG in young CAD patients with age less than 50 years and triple vessel disease is associated with a significantly lower major adverse cardiovascular event as compared to PCI.16 The patients who develop CAD at a young age also develop complications related to it at an early age. The early onset of heart failure, recurrent arrhythmias, stroke, transient ischemic attack, recurrent hospitalization, repeat revascularization, and medication complication has a great financial and psychological impact on the patient life. Also, the early onset of chronic illness leads to the loss of productive years of patient life. The presence of early CAD significantly increases the number of morbidity years for the patient and increases the financial burden on the overall population.
The rate of ISR following DES implantation in our study was 5.63% which is similar to as observed in studies without angiographic surveillance.17 The mean duration to develop ISR in our study population was similar to the previously reported study.18 DM was significantly associated with the development of restenosis in our study. Previously conducted studies had clearly shown that the DM is the most consistent and strongest risk factor associated with the development of ISR.19 The presence of DM is associated with increased blood viscosity, increased shear stress and smooth muscle cell proliferation. Increased effect of stimulatory growth factors like insulin-like growth factors on vascular smooth muscle cells leads to an increased risk of neointimal proliferation and restenosis.
In our study, on multiple Cox-regression analyses, there was no association between increased risks of ISR with the number of the stent deployed per patient, and the average length of the stent deployed per patient. Previous studies had shown the increased risk of restenosis with an increased number of the stent and total length of the stent.20 The result of our study could be due to the small sample size, retrospective nature of the study and the absence of quantitative coronary angiogram data for our study population.
We also found that the development of restenosis is strongly associated with the location of the stent in LAD territory which is similar to previously conducted studies.20 On multiple regressions analysis, restenosis was also found to be strongly associated with the periprocedural complication during the time of PCI. In our study, patients with DM developed more ISR and they had poor event-free survival for the development of ISR as compared to patients without DM which is similar to previously reported studies.21 However, among ISR patients, there was no difference among DM patients vs. patients without DM about time to restenosis. We think this could be due to small sample size and a very less number of ISR in our study population.
The rate of ST in our study was 4.4%. The higher than expected rate of ST in our study could be due to the implantation of the first-generation stent in majority of our study population and discontinuation of antiplatelet therapy by all the patients who developed ST. Although the risk of the ST in our study is higher than the previously reported studies, however, it was nearly similar to the study in which patient discontinued their antiplatelet.22 According to ACC/AHA, both presences of DM and proximal LAD are the major factors for determining appropriate strategies between CABG vs. PCI for revascularisation in CAD patients.23 The main reason for this recommendation is the difference with regards to the rate of TLR and mortality between PCI vs. CABG. The current study clearly shows the increased risk of restenosis in patients having both DM and LAD as risk factors as compared to patients without these risk factors. Also, nearly 10% of ISR patients in our study presented with STEMI. Also, we observed an increased risk of very late stent thrombosis after stent implantation. All of these findings point toward the consideration of appropriate revascularization strategies (PCI vs. CABG) in this high-risk group of patients. However, further study is needed to confirm these findings.
In our study, we observed that patients continue to have very late stent thrombosis after DES implantation. ACC/AHA had recommended at least one year dual antiplatelet after DES implantation in patients with ACS and at least 6 months inpatient with stable ischemic heart diseases.24 An increased risk of ST in our study points toward a reconsideration of this strategy in the context of our population. However, our data is not sufficient. Further studies are needed to look for ST with the use of the newer generation of the stent. Also, because of the increased risk of late and very late stent thrombosis, there is a need for long-term follow-up after DES implantation and continuous education of patients regarding the need to continue antiplatelet therapy.
The major limitation of our study was the retrospective nature of the study. Prevalent use of first-generation DES which is now obsolete is another major drawback of our study. The absence of routine angiogram along with quantitative coronary angiogram data (lesion length, reference vessel diameter, luminal gain, luminal loss) in the study population is a major limitation. The result of this study can be extrapolated to our population but still, a large prospective cohort study is needed.
ConclusionIn our study, the rate of restenosis after DES implantation was 5.63%. The rate of TLR in the study population was 5.63%. ISR is significantly associated with DM, the presence of the stent in LAD location and periprocedural complications. Our study for the first time is providing the incidence of ISR and risk factors associated with it in a large sample size from our population. In our study, we had calculated the median time to develop restenosis in the overall population, in DM patients and also in patients without DM which was not available from previously conducted studies to our best knowledge.
FundingNone declared.
Conflict of interestThe authors have no conflicts of interest to declare.