To assess the relationship between vitamin D deficiency and severity of coronary artery disease using multislice CT coronary angiography.
Methods100 patients diagnosed with coronary artery disease during multislice CT coronary angiography were subjected to full evaluation of coronary artery disease severity followed by measurement of serum vitamin D level.
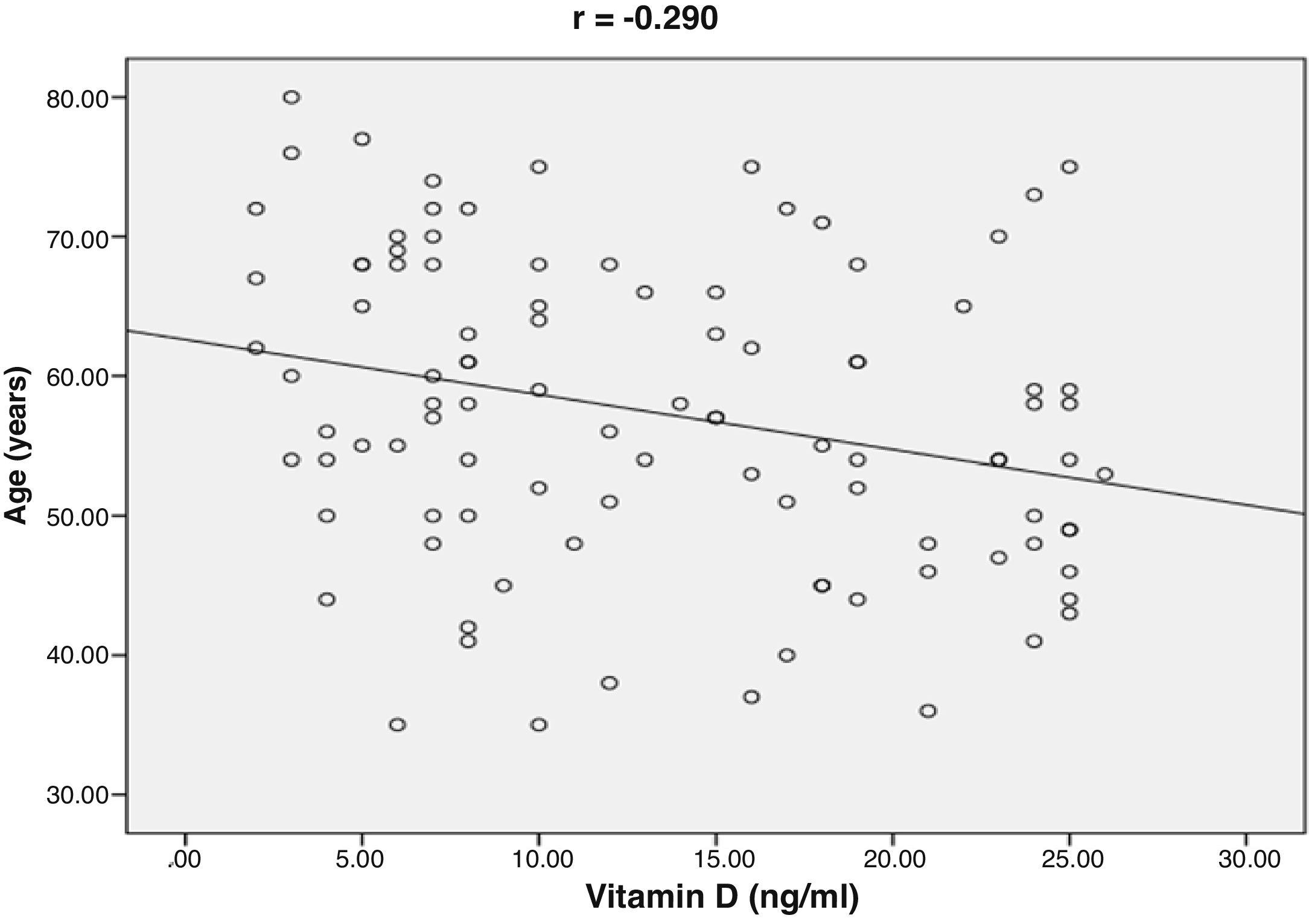
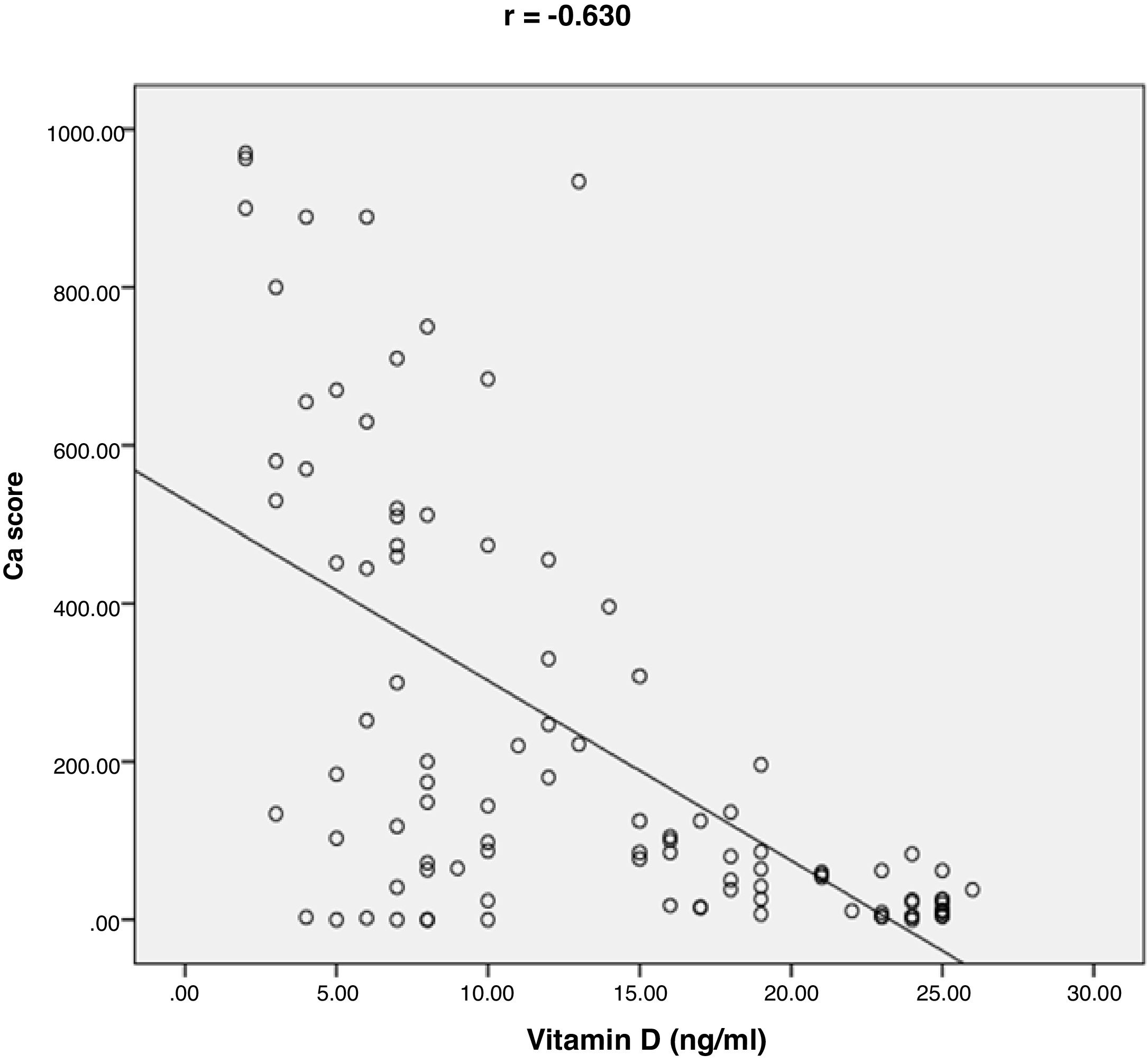
ResultsThe mean value of serum vitamin D level was 13.35±7.49ng/ml. 76% of the patients had vitamin D deficiency (<20ng/ml). 41% of the patients had single vessel disease, 28% had two vessel diseases, and 31% had multi-vessel disease. Patients with vitamin D deficiency had higher degree of coronary stenosis, higher coronary Ca score (p<0.001) and higher number of affected vessels compared with normal vitamin D level subgroup (p<0.001). Vitamin D level showed a significant negative correlations with age (r=−0.290, p=0.003), coronary Ca score (r=−0.630, p<0.001) and severity of coronary lesions. Multivariate linear regression analysis showed that dyslipidemia and vitamin D level were independent predictors of percent severity of coronary stenosis.
ConclusionIn addition to traditional cardiovascular risk factors, vitamin D deficiency looks to be independent predictor of coronary artery disease severity including percent stenosis, number of the affected vessels as well as degree of coronary calcification.
Evaluar la relación entre el déficit de vitamina D y la gravedad de la arteriopatía coronaria mediante angio-TAC coronaria multicortes.
MétodosCien pacientes con diagnóstico de arteriopatía coronaria durante la realización de angio-TAC multicortes fueron sometidos a evaluación completa de la gravedad de la enfermedad tras la medición del nivel de vitamina D sérico.
ResultadosEl valor medio del nivel de vitamina D sérico fue de 13,35 ± 7,49 ng/ml. El 76% de los pacientes tenían déficit de vitamina D (<20 ng/ml). El 41% de los pacientes tenía afectado un único vaso, y el 28% tenía afectados dos vasos, y el 31% tenía afectados múltiples vasos. Los pacientes con déficit de vitamina D tenían un mayor grado de estenosis coronaria, mayor puntuación de Ca coronario (p < 0,001) y un mayor número de vasos afectados en comparación con el subgrupo con un nivel normal de vitamina D (p < 0,001). El nivel de vitamina D reflejó correlaciones negativas significativas con la edad (r = −0,290, p = 0,003), puntuación de Ca coronario (r = −0,630, p < 0,001) y gravedad de las lesiones coronarias. El análisis de regresión lineal multivariante reflejó que la dislipidemia y el nivel de vitamina D eran factores predictivos independientes del porcentaje de gravedad de la estenosis coronaria.
ConclusiónAdemás de los factores tradicionales de riesgo cardiovascular, el déficit de vitamina D parece ser un factor predictivo independiente de la gravedad de la arteriopatía coronaria incluyendo el porcentaje de estenosis, el número de vasos afectados y el grado de calcificación coronaria.
Vitamin D deficiency is a common health problem prevalent across the world and more severe in elderly patients.1 Vitamin D deficiency has been usually related to bone health,2 however recent studies show strong relation between vitamin D deficiency and cardiovascular disease, high blood pressure, insulin resistance, heart failure and disabling strokes.3,4 The protective effect of vitamin D against cardiovascular diseases can be explained by several mechanisms like the effect of the vitamin D on the renin–angiotensin system, vessel compliance, blood pressure, parathyroid hormone level and also glycemic control. Vitamin D has added anti-inflammatory effects and prevents cholesterol removal by macrophage and formation of foam cells. An inverse relationship has been seen between vitamin D level and coronary artery calcification.5 Large epidemiological studies have demonstrated that deficiency of vitamin D is considered as a cardiovascular risk,6 increasing vascular stiffness, predisposing for accelerated atherosclerosis,7 and subsequent cardiovascular events.8 Several studies have demonstrated strong relationship between deficiency of vitamin D and coronary artery disease (CAD) severity.9,10 Coronary computed tomography angiography (CTA) was introduced as a noninvasive method for CAD assessment. CTA has been suggested as a reliable noninvasive tool to exclude CAD because of its high sensitivity.11 The aim of our study was to evaluate the association between deficiency of vitamin D and (CAD) severity using multislice CT coronary angiography.
Methods100 patients diagnosed to have (CAD) during multislice CT coronary angiography were randomly selected regardless their baseline characteristics from radiology department, during period between October 2017 and August 2019 through an observational, cross-sectional study. The study was approved by the Faculty of medicine (Beni-Suef University) ethical committee. Written informed consent was obtained from every patient to participate in the study. Patients on regular vitamin D supplementations and those with coronary artery Ca score (CAC) >1000 by Agatston's score were excluded from the study.
Study designPatients referred for multislice CT coronary angiography were subjected to contrast enhanced coronary angiography using multi-slice CT, GE Revolution (256-slices) after IV contrast administration of (75ml) of non-ionic contrast media. This was followed by rapid acquisition of constructive ultra-thin sections through the heart to evaluate the coronary arteries. Post processing was done using a dedicated work station (ADW 4.7). 3D curved multi-planar reformatted images were created by the interpreting physician utilizing maximum intensity projection technique. Patients diagnosed with (CAD) during multislice CT coronary angiography were included in the study and subjected to full evaluation of coronary artery disease severity including percent severity of coronary stenosis and number of affected vessels. Cross-sectional images were acquired in the most severe part of stenosis and in the proximal and distal reference sites. The minimal lumen diameter was measured in the previous 3 images. The reference vessel diameter was measured by estimating the average minimal lumen diameters of the proximal and distal sites. The percent coronary stenosis was estimated by subtracting the reference diameter from the minimal lumen diameter that was divided by the reference diameter. Severity of coronary stenosis was determined as follow: 0–24%: minimal stenosis, 25–49%: mild stenosis, 50–69%: moderate stenosis, and more than 70%: severe stenosis. Segments selected for CAD analysis were as followings: Left main: ostium, mid shaft and distal, Left anterior descending artery (LAD): proximal, mid and distal, Left circumflex artery (LCX): proximal and distal, Right coronary artery (RCA): proximal, mid and distal. Small distal vessels less than 2mm were excluded from angiographic analysis. The total calcium load in the coronary arteries was manually evaluated by planimetry according to the scoring algorithm of Agatston et al.12 Based on Agatston score, patients were classified into 5 categories,13 0 calcium score: no evidence of CAD, 1–10 calcium score: minimal evidence of CAD, 11–100 calcium score: mild evidence of CAD. 101–400 calcium score: moderate evidence of CAD, and over 400 calcium score: extensive evidence of CAD. All patients diagnosed with CAD during multi-slice CT examination were subjected to the following: (A) full history taking including: age, gender and risk factors for (CAD) as systemic hypertension (HTN), diabetes mellitus (DM), dyslipidemia, obesity and smoking. (B) Clinical examination, 12 leads ECG and echocardiography. (C) Laboratory investigations including: (1) serum vitamin D measurement by liquid chromatography–tandem mass spectrometry to detect the association between serum level of 25 hydroxyvitamin D3 (25(OH)D3) deficiency and severity of coronary artery disease (CAD). Normal value of vitamin D was defined according to The Endocrine Society Guidelines as serum 25(OH)D3 level of 20–50ng/ml based on analysis of observational studies of vitamin D and various health outcomes. Vitamin D deficiency was defined as serum 25(OH)D3 level of less than 20ng/ml.14 (2) Renal function tests: serum urea and creatinine. (3) Fasting blood sugar (FBS), and HbA1C%. (4) Complete lipid profile.
Statistical analysisStatistical analysis was done using statistical package for social sciences (SPSS), computer software (version22), IBM software, USA. We divided all patients into two subgroups according to vitamin D level (either normal or deficient). Data were proven to be abnormally distributed using Kolmogorov test. Comparison of numerical variables between study subgroups was done using non-parametric unpaired Mann–Whitney U test. Numerical variables were expressed as mean±standard deviation (SD), or (number of cases) and percentages when appropriate. Qualitative data were expressed as frequency and percentage. Chi-square (χ2) test was used in order to compare between two qualitative measures. A p value <0.05 was considered statistically significant. Spearman's correlation was used to assess the degree of correlation between two sets of variables. All significant variables associated with severity of CAD were introduced in Multivariate linear regression analysis model to find independent predictors of CAD severity. Power of sample size was estimated using G*power software (3.1.9.2) based on effect size, overall type I error rate (α) ≤0.05, a total of 100 patients was excellent to achieve a power of (95–100%).
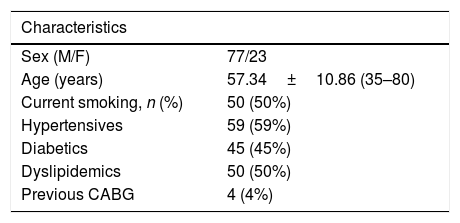
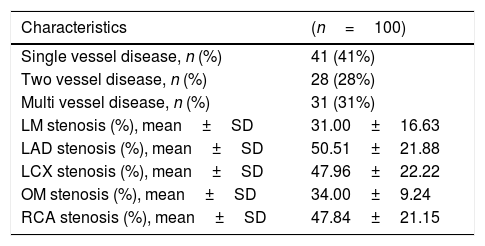
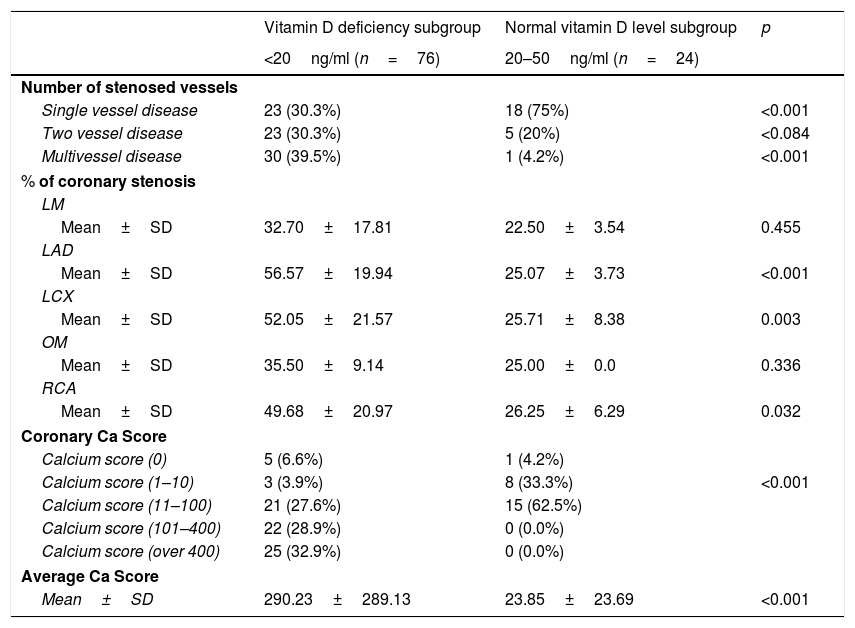
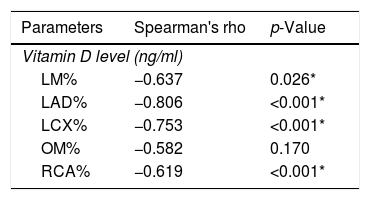
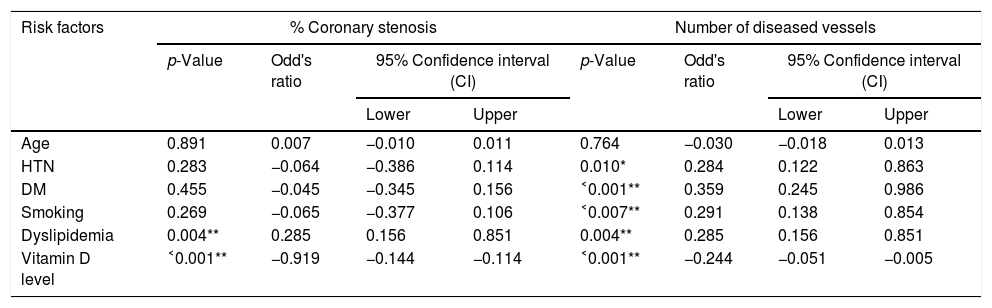
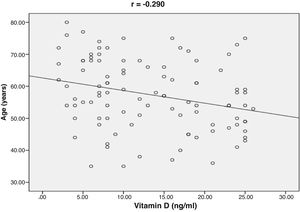
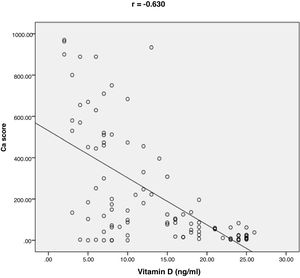
ResultsThe baseline characteristics of all subjects under study are shown in (Table 1). Our study included 77 men and 23 women. Age ranged from 35 to 80 years, mean age was 57.34±10.86 years. Regarding lab results, the mean value of serum vitamin D level was 13.35±7.49ng/ml. 76% of the patients had vitamin D deficiency <20ng/ml. Patients with vitamin D deficiency were significantly older than those with normal vitamin D level (p=0.026). No significant difference regarding sex distribution between those with and without vitamin D deficiency (p=0.410). Vitamin D level showed a significant negative correlation with age (r=−0.290, p=0.003) (Fig. 1). CT angiographic results were summarized in (Table 2). Higher proportions of patients with vitamin D deficiency had single or multi-vessel disease (p<0.001) and higher percentage of coronary stenosis compared with those with normal vitamin D level. 31% of the patients had multi-vessels disease while 41% of the patients had single vessel disease. 25% of our patients had a (CAC) over 400. Patients with vitamin D deficiency had higher (CAC) compared with those with normal vitamin D level (Table 3). Vitamin D level showed a significant negative correlation with (CAC) (r=−0.630, p<0.001) (Fig. 2) and severity of coronary lesions (Table 4). Multivariate linear regression analysis showed that dyslipidemia and vitamin D level were independent predictors of percent severity of coronary stenosis. Vitamin D level in addition to Diabetes Mellitus, smoking, hypertension and dyslipidemia were found to be independent predictors of number of diseased vessels (Table 5).
Baseline characteristics of all patients under study (n=100).
| Characteristics | |
|---|---|
| Sex (M/F) | 77/23 |
| Age (years) | 57.34±10.86 (35–80) |
| Current smoking, n (%) | 50 (50%) |
| Hypertensives | 59 (59%) |
| Diabetics | 45 (45%) |
| Dyslipidemics | 50 (50%) |
| Previous CABG | 4 (4%) |
M: male; F: female; CABG: coronary artery by-pass grafting.
CT angiographic results.
| Characteristics | (n=100) |
|---|---|
| Single vessel disease, n (%) | 41 (41%) |
| Two vessel disease, n (%) | 28 (28%) |
| Multi vessel disease, n (%) | 31 (31%) |
| LM stenosis (%), mean±SD | 31.00±16.63 |
| LAD stenosis (%), mean±SD | 50.51±21.88 |
| LCX stenosis (%), mean±SD | 47.96±22.22 |
| OM stenosis (%), mean±SD | 34.00±9.24 |
| RCA stenosis (%), mean±SD | 47.84±21.15 |
LM: left main; LAD: left anterior descending; LCX: left circumflex; OM: obtuse marginal; RCA: right coronary artery.
Comparison between patients with vitamin D deficiency and those with normal vitamin D level regarding CT angiographic results.
| Vitamin D deficiency subgroup | Normal vitamin D level subgroup | p | |
|---|---|---|---|
| <20ng/ml (n=76) | 20–50ng/ml (n=24) | ||
| Number of stenosed vessels | |||
| Single vessel disease | 23 (30.3%) | 18 (75%) | <0.001 |
| Two vessel disease | 23 (30.3%) | 5 (20%) | <0.084 |
| Multivessel disease | 30 (39.5%) | 1 (4.2%) | <0.001 |
| % of coronary stenosis | |||
| LM | |||
| Mean±SD | 32.70±17.81 | 22.50±3.54 | 0.455 |
| LAD | |||
| Mean±SD | 56.57±19.94 | 25.07±3.73 | <0.001 |
| LCX | |||
| Mean±SD | 52.05±21.57 | 25.71±8.38 | 0.003 |
| OM | |||
| Mean±SD | 35.50±9.14 | 25.00±0.0 | 0.336 |
| RCA | |||
| Mean±SD | 49.68±20.97 | 26.25±6.29 | 0.032 |
| Coronary Ca Score | |||
| Calcium score (0) | 5 (6.6%) | 1 (4.2%) | |
| Calcium score (1–10) | 3 (3.9%) | 8 (33.3%) | <0.001 |
| Calcium score (11–100) | 21 (27.6%) | 15 (62.5%) | |
| Calcium score (101–400) | 22 (28.9%) | 0 (0.0%) | |
| Calcium score (over 400) | 25 (32.9%) | 0 (0.0%) | |
| Average Ca Score | |||
| Mean±SD | 290.23±289.13 | 23.85±23.69 | <0.001 |
LM: left main; LAD: left anterior descending; LCX: left circumflex; RCA: right coronary artery; OM: obtuse marginal.
Multivariate linear regression analysis for independent predictors of percent severity of coronary stenosis and number of diseased coronary vessels.
| Risk factors | % Coronary stenosis | Number of diseased vessels | ||||||
|---|---|---|---|---|---|---|---|---|
| p-Value | Odd's ratio | 95% Confidence interval (CI) | p-Value | Odd's ratio | 95% Confidence interval (CI) | |||
| Lower | Upper | Lower | Upper | |||||
| Age | 0.891 | 0.007 | −0.010 | 0.011 | 0.764 | −0.030 | −0.018 | 0.013 |
| HTN | 0.283 | −0.064 | −0.386 | 0.114 | 0.010* | 0.284 | 0.122 | 0.863 |
| DM | 0.455 | −0.045 | −0.345 | 0.156 | ˂0.001** | 0.359 | 0.245 | 0.986 |
| Smoking | 0.269 | −0.065 | −0.377 | 0.106 | ˂0.007** | 0.291 | 0.138 | 0.854 |
| Dyslipidemia | 0.004** | 0.285 | 0.156 | 0.851 | 0.004** | 0.285 | 0.156 | 0.851 |
| Vitamin D level | ˂0.001** | −0.919 | −0.144 | −0.114 | ˂0.001** | −0.244 | −0.051 | −0.005 |
HTN: hypertension; DM: diabetes mellitus.
We performed the present study to assess the association between vitamin D deficiency and coronary artery disease severity using multislice CT coronary angiography. Our study results revealed that patients with vitamin D deficiency had higher number of diseased vessels (p<0.001), higher percentage of coronary stenosis. 31% of the patients had multi-vessels disease while 41% of the patients had single vessel disease. There was a significant negative correlation between vitamin D level and percent severity of coronary stenosis.
In agreement with our findings, Moradi and colleagues found a significant lower vitamin D level in patients with multi vessel disease compared with patients with single-vessel disease or normal coronaries.15 Our results were in agreement with the Korean Longitudinal Study on Health and Aging, low vitamin D3 levels were associated with increased prevalence of a more than 50% coronary artery stenosis as determined by cardiac CT angiography.16 Seckin Satilmis et al. conducted a single center, observational study on 208 patients, under age of 45 years. They were subjected to cardiac CT angiography, the study showed an inverse association between serum level of vitamin D and the severity of coronary atherosclerosis as determined by CT angiography.17 Akin et al.,18 reported that vitamin D insufficiency was associated with increased severity of coronary artery stenosis. There are some mechanisms proposed to explain the relation between 25(OH)D3 deficiency and coronary atherosclerosis. 25(OH)D3 receptor has been found in most tissues and cells, which include vascular smooth muscle cells,19 macrophages,20 cardiomyocytes,21 endothelium,22 and lymphocytes 23 25(OH)D stimulates production of prostacyclin by vascular smooth muscle cells, which prevents thrombus formation, cell adhesion, and smooth muscle cell proliferation.24 Vascular smooth muscle cells and endothelial cells express receptors for 25-hydroxyvitamin D3 (25(OH)D3), the major circulating metabolite of vitamin D3, which can convert it to 1,25-dihydroxyvitamin D3 (1,25(OH)2D).25 Al Mheid et al., 26 reported that 25(OH)D3 insufficiency was accompanied with increased endothelial dysfunction and arterial stiffness in asymptomatic population. Oh et al.,27 reported that active 25(OH)D3 inhibits foam-cell synthesis by reducing macrophage cholesterol uptake in diabetics. Another mechanism explaining the effects of 25(OH)D3 and its metabolites on atherosclerosis may be related to their anti-inflammatory actions. The Ludwigshafen Risk and Cardiovascular Health study28 demonstrated an increased inflammatory reaction in patients with low 25(OH)D3 status.
25% of our patients had (CAC) over 400. Patients with vitamin deficiency had higher coronary Ca score (p<0.001) compared with normal vitamin D level subgroup. CAC score was negatively correlated with vitamin D level (r=−0.630; p<0.001). Our results were in agreement with Boer et al. They assessed the association between vitamin D deficiency and coronary Ca score (CAC) using multi slice CT in a cohort study. They found that low vitamin D level was associated with increased risk of developing incident CAC.29 Moradi et al. conducted a cross-sectional study on 180 patients to investigate the relation between CAC score and vitamin D level. The results showed a significant higher CAC score among patients with vitamin D deficiency compared to patients with vitamin D insufficiency or normal vitamin D level. CAC score was negatively correlated with vitamin D level.15 Lim and colleagues found a significantly negative correlation between CAC score and vitamin D level. Patients with vitamin D deficiency were more likely to have higher CAC score.16 Deficiency of vitamin D levels has been associated also with inflammation, higher coronary artery calcium scores, increased platelet volume, increased vascular stiffness30,31 and higher circulating concentrations of matrix metalloproteinase-9, which affects vascular wall remodeling and calcification.32 Vitamin D has a major role in maintaining endothelial integrity. The mechanism of vitamin D deficiency in accelerating vascular calcification can be explained by the effect of pro-inflammatory factors predisposing to endothelial stress and dysfunction. This can be the stimulus for calcification.33 In contrast, Ho and colleagues conducted a prospective study on 1131 consecutive individuals to assess the effect of low vitamin D level on CAC score. They found non-significant association between vitamin D3 levels and coronary calcium score, or severity of coronary stenosis.34
Our study had some limitations. Firstly, it was an observational and cross-sectional study, consequently study population was heterogenous with wide range of age (35–80 years), little representation by sex (only 23 patients were women). Secondly presence of a control arm with normal coronaries would be helpful to clarify the relationship between vitamin D deficiency and severity of coronary artery disease but we are limited by a relative costly CT coronary examination and vitamin D measurement done for 100 patients without any grant from any funding agency.
ConclusionIn addition to traditional cardiovascular risk factors, vitamin D deficiency looks to be independent predictor of coronary artery disease severity including percent stenosis, number of the affected vessels as well as degree of coronary calcification
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestsThe authors have no conflict of interests to declare