Modified citrus pectin (MCP) is used as a nutritional supplement that inhibits galectin-3 activity, a central player in the cardiac damage associated with different pathological situations. In fact, we have previously observed that MCP improved cardiac function in obese infarcted rats that was associated with a reduction in cardiac fibrosis. Therefore, the aim of the present study was to further explore whether this effect could involve the modulation of gene expression of ECM components and their mediators as well as whether it could affect another two mechanisms involved in cardiac damage: mitochondrial dynamics and autophagic flux.
MethodsMale Wistar rats were fed an atherogenic diet with a high content of saturated fat (35%). MI was induced by the ligation of left anterior descendant (LAD) coronary artery 6 weeks after and MCP (100mg/kg/day) or vehicle were administered for 4 weeks more. A group of rats fed a standard diet (5.3% fat) and subjected to a sham operation was used as controls.
ResultsObese infarcted animals presented an increase in cross-linked collagen that was not affected by the administration of galectin-3 inhibitor. However, MCP reduced the increase in gene expression observed in obese infarcted rats of ECM components and mediators (collagen I, fibronectin, transforming growth factor-β and connective tissue growth factor), of components of endoplasmic reticulum stress (binding immunoglobulin protein, CCAAT-enhancer-binding homologous protein and activating transcription factor 4), of oxidative stress mediator (NADPH oxidase-4) and normalized those of the interleukin 33/ST2 system. MCP is also able to increase the levels of the mitochondrial protein Dynamin-1-like and those of both proteins involved in autophagic flux (p62 and LC3) that were reduced by the myocardial ischemia in the context of obesity.
ConclusionsThe data show that the beneficial effect of the nutritional supplement MCP on the cardiac consequences associated with myocardial ischemia in the context of obesity could rely on its capacity to inhibit galectin-3 and to consequently modulate different downstream mechanisms, including inflammation, ER stress, oxidative stress, autophagy and mitochondrial function, which can facilitate fibrosis and cardiac remodeling in this pathological context.
La pectina cítrica modificada (MCP) es un suplemento nutricional que inhibe la actividad de la galectina-3, que juega un papel importante en el daño cardíaco asociado con diferentes situaciones patológicas. Hemos observado previamente que la MCP mejora la función cardíaca en ratas obesas infartadas, mejora que se asoció con una reducción de la fibrosis cardíaca. Por ello, el objetivo del presente estudio fue explorar más a fondo si este efecto pudiera implicar la modulación de la expresión génica de los componentes de la matriz extracelular y sus mediadores, así como afectar a otros 2 mecanismos involucrados en el daño cardíaco: la dinámica mitocondrial y el flujo autofágico.
MétodosRatas Wistar macho fueron alimentadas con una dieta aterogénica con un alto contenido de grasa saturada (35%). El infarto de miocardio se indujo mediante la ligadura de la arteria coronaria descendiente anterior izquierda 6 semanas después, y se administró MCP (100mg/kg/día) o vehículo durante 4 semanas más. Un grupo de ratas alimentadas con una dieta estándar (5,3% de grasa) y sometidas a una operación simulada se utilizó como grupo control.
ResultadosLos animales obesos infartados presentaron un aumento del colágeno entrecruzado que no se vio afectado por la administración del inhibidor de la galectina-3. Sin embargo, la MCP redujo el aumento de la expresión génica observada en ratas obesas infartadas de los componentes y mediadores de la matriz extracelular (colágeno I, fibronectina, factor de crecimiento transformante-ß y factor de crecimiento del tejido conectivo), de los componentes del estrés del retículo endoplasmático (RE, por sus siglas en inglés), proteína de unión a inmunoglobulina, proteína homóloga de unión al potenciador CCAAT y factor de transcripción activador 4, del mediador del estrés oxidativo (NADPH oxidasa-4) y normalizó los del sistema interleucina 33/ST2. La MCP también fue capaz de aumentar los niveles de la proteína mitocondrial Dynamin-1-like y los de las 2 proteínas implicadas en el flujo autofágico (p62 y LC3), que se redujeron por la isquemia miocárdica en el contexto de la obesidad.
ConclusionesLos datos muestran que el efecto beneficioso del suplemento nutricional MCP sobre las consecuencias cardíacas asociadas a la isquemia miocárdica en el contexto de la obesidad podría depender de su capacidad de inhibir la galectina-3 y la consiguiente modulación de diferentes mecanismos incluidos la inflamación, el estrés del RE, el estrés oxidativo, la autofagia y la función mitocondrial que pueden facilitar la fibrosis y la remodelación cardíaca en este contexto patológico.
The consumption of dietary supplements is a current practice worldwide, which can help to get adequate amounts of essential nutrients but also can offer health benefits. They contain minerals, herbs, amino acids, enzymes, fiber and many other ingredients. Pectin is a type of soluble fiber found in fruits and vegetables, particularly including citrus peels. It is commonly used as a thickening agent in jams and jellies, however due to its properties as a fiber, pectins are used as dietary supplements, being able to regulate the viscosity of digestive contents and making slower the release of glucose into the bloodstream.1 Modified citrus pectin (MCP) is a complex water-soluble and indigestible polysaccharide rich in β-galactose2 derived from citrus pectin, which is fragmented in low molecular weight–low esterification products by pH and temperature changes, being now well absorbed from the small intestinal epithelium into the circulation.3 Indeed, due to the health benefits shown in different studies, MCP is used as a potential, safe and non-toxic treatment,4 its consumption being easier in regular diets as part of snacks or baked goods.1
In addition to the digestive benefits, MCP is able to modulate the bioactivity of galectin-3 by binding to the carbohydrate recognition domains of the molecule.3 Galectin-3 is a protein from the lectin family that can exert different functions depending on its location in cells and it is involved in different biological processes by regulating short-distance signaling (cell–cell and cell–extracellular matrix (ECM) interaction), gene expression and the transcription of different proteins among others.5,6 At cardiac level, galectin-3 acts as a pro-fibrotic, pro-oxidant and pro-inflammatory mediator playing a relevant role in the development of cardiac damage associated with different pathological situations, including myocardial infarction, coronary artery disease and obesity.2,5,7,8 In agreement with this, MCP administration is associated with an improvement in the cardiac consequences associated with obesity, heat failure, hyperaldosteronism and hypertension.8–10 In fact, in a previous study we have found that MCP was able to improve the alterations observed in cardiac function in obese infarcted rats that was accompanied by a reduction in protein expression of components and mediators of ECM.2 Therefore, the aim of this study was to go deeper into the effect of MCP in the cardiac consequences associated with myocardial ischemia in the context of obesity by evaluating whether MCP can reduce ECM acting at gene level, as well as whether its beneficial effects could involve a modulation of autophagy flux and mitochondrial dynamics, two mechanisms involved in cardiac damage.11,12
MethodsAnimal modelMyocardial infarction (MI) was induced by ligation of the left anterior descendant (LAD) coronary artery in male Wistar rats of 150g (Envigo RMS, S.L., Barcelona, Spain), which were fed a proatherogenic diet with high-saturated fat content (HFD; 35% fat; Envigo Teklad no. TD.03307, Haslett, MI, USA) for 10 weeks. The ligation was performed at the sixth week, once significant differences in the body weight were observed between HFD animals and a reference group (CT), which included rats fed with a standard diet (5.3% fat; Lasvendi Rod 18-A, Soest, Germany) and subjected to a sham operation (the same surgical procedure without fastening of the suture that passes through the LAD). The obese rats with MI received for the following 4 weeks either the vehicle or MCP (100mg/kg/day; p.o.), the inhibitor of galectin-3 activity. The dose of MCP treatment was based on previous data.8
The body weight was measured once a week and cardiac structure was evaluated at the end of the evolution period by transthoracic echocardiography (TTE) with an GE Vivid-I (General Electric Healthcare, Boston, MA, USA) portable device using an 12S-RS transducer. The images were processed with the Echopack software v. 201 (General Electric Healthcare, Boston, MA, USA). At the end of the experiment heart and the different fat pads (epididymal, lumbar, and mesenteric adipose tissue) were collected. Adiposity index was calculated as follows: sum of fat pads/[(body weight−fat pad weight)×100]. Ethical approval for the animal study was obtained from the Animal Care and Use Committee of Universidad Complutense de Madrid and Dirección General de Medio Ambiente, Comunidad de Madrid, Spain (PROEX 349.3/21).
Morphological and histological evaluationCardiac tissue samples were dehydrated, embedded in paraffin, and cut into 4μm thick sections and stained with picrosirius red and viewed with polarized light under dark-field optics to detect the birefringence of collagen fibers. The area of cross-linked collagen was identified as the ratio between of cross-linked collagen to the total collagen content after excluding the vessel area from the region of interest. 10–15 fields per sample were analyzed with a 40× objective under transmitted light microscopy (Leica DM 2000; Leica AG, Wetzlar, Germany). Quantitative analysis was performed using an analysis system (Leica LAS 4.3; Leica AG, Wetzlar, Germany).
Western blot analysisCardiac samples were homogenized, and 40μg total cardiac proteins were extracted, separated by SDS-PAGE on 4–20% polyacrylamide gels and transferred to Hybond-c Extra nitrocellulose membranes (Hybond-P; Amersham Biosciences, Piscataway, NJ, USA) with the Trans-Blot Turbo Transfer System. Membranes were probed with primary antibodies for all the following: Dynamin-1-like protein (DRP1, Abcam; Cambridge, UK; dilution 1:500), LC3I/II (Cell Signaling Technology, Danvers, MA, USA; dilution 1:1000), mitofusin 1 (MFN1, Abcam; Cambridge, UK; dilution 1:1000) and p62 (Abnova, Taiwan; dilution 1:1000). Stain free was used as loading control. The signals were detected using the ECL system (Millipore, Burlington, MA, USA). The results are expressed as n-fold increases over the values of the control group in arbitrary densitometric units.
Retrotranscription and real-time PCRTotal RNA was isolated using Trizol Reagent (Fisher Scientific, Waltham, MA, USA) and was reverse-transcribed into cDNA using the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific Inc, Waltham, MA, USA). Quantitative PCR analysis was performed with SYBR green PCR technology (Fisher Scientific, Waltham, MA, USA) and specific oligonucleotides (Table S1). Quantification of mRNA levels was performed by real-time PCR using the 2−ΔΔCt method. Data were normalized to hypoxanthine phosphoribosyltransferase (Hprt).
Statistical analysisVariables are expressed as mean±standard error of the mean (SEM). The normality of distributions was verified by Kolmogorov–Smirnov. Specific differences between groups were analyzed using a one-way analysis of variance followed by a Newman–Keuls test. Either Pearson or Spearman correlation analyses were used to examine the associations among different variables according to whether they were normally distributed. A value of p<0.05 was used as the cut-off value for defining statistical significance. The data analysis was performed using the statistical programs GraphPad Prism 8 (San Diego, CA, USA) and SPSS version 25.0 (SPSS Inc., Chicago, IL, USA).
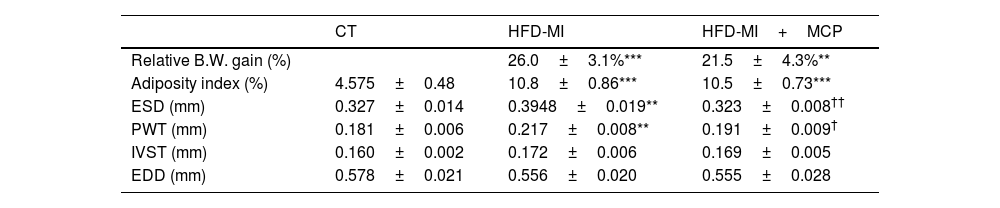
ResultsInfarcted animals fed a HFD showed a higher relative increase in body weight at the end of the study as compared with the control group, which was similar in MCP-treated and -untreated animals (Table 1). An increase in adiposity index was also observed in HFD-MI as compared with CT rats, which was not modified by the administration of MCP (Table 1).
Relative body weight gain, adiposity index and echocardiographic parameters in rats subjected to a sham operation (CT) and obese rats submitted to myocardial infarction treated with vehicle (HFD-MI) or with modified citrus pectin (HFD-MI+MCP; 100mg/kg/day).
| CT | HFD-MI | HFD-MI+MCP | |
|---|---|---|---|
| Relative B.W. gain (%) | 26.0±3.1%*** | 21.5±4.3%** | |
| Adiposity index (%) | 4.575±0.48 | 10.8±0.86*** | 10.5±0.73*** |
| ESD (mm) | 0.327±0.014 | 0.3948±0.019** | 0.323±0.008†† |
| PWT (mm) | 0.181±0.006 | 0.217±0.008** | 0.191±0.009† |
| IVST (mm) | 0.160±0.002 | 0.172±0.006 | 0.169±0.005 |
| EDD (mm) | 0.578±0.021 | 0.556±0.020 | 0.555±0.028 |
B.W.: body weight, ESD: end-systolic diameter, PWT: posterior wall thickness, IVST: interventricular septum thickness and EDD: end-diastolic diameter. Values represent the mean±SEM of 7–10 animals.
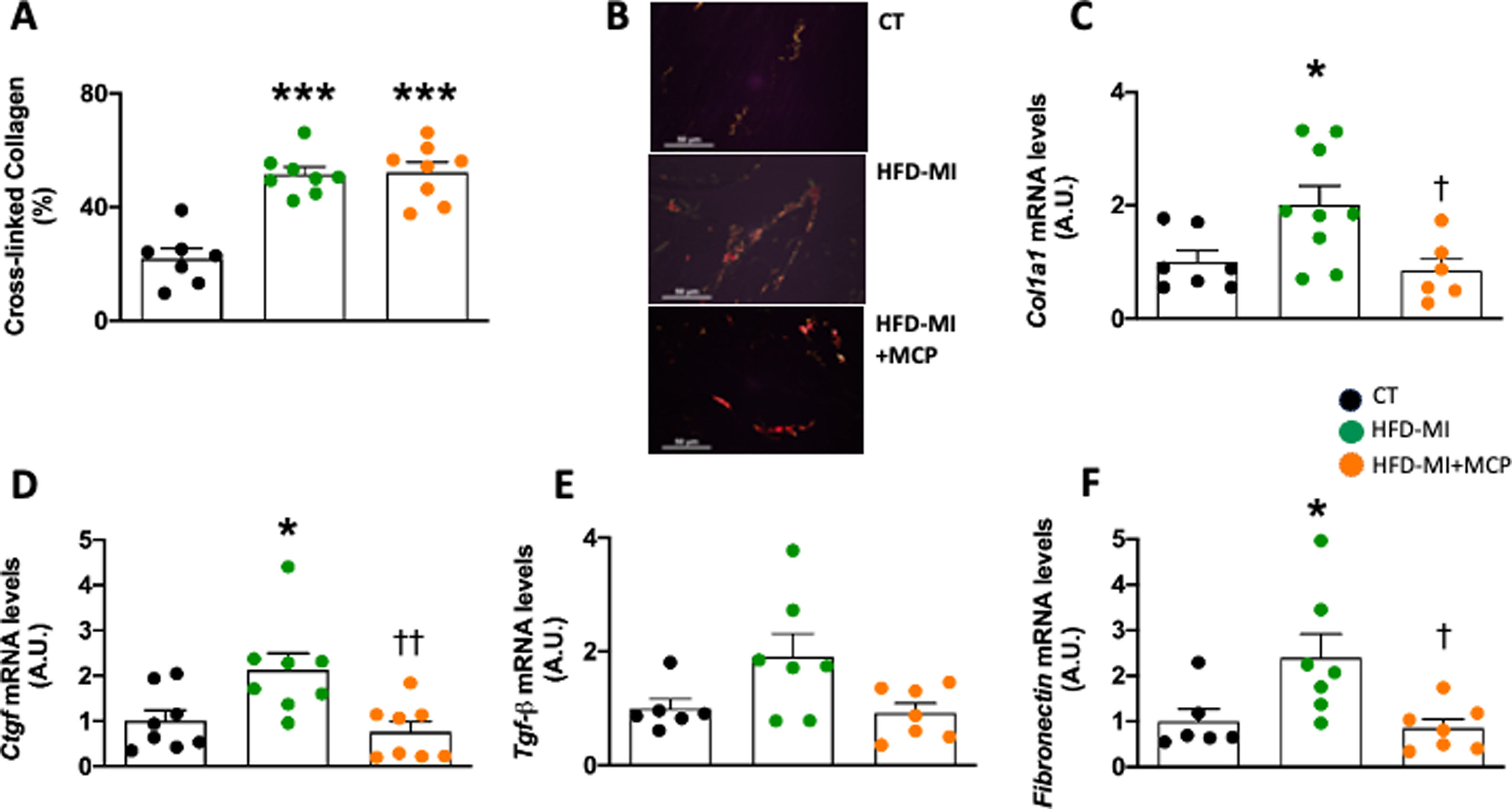
Obese infarcted rats also showed an increase in relative heart weight, suggesting cardiac hypertrophy, and higher galectin-3 gene expression than in control animals, as we previously described.2 The galectin-3 activity inhibitor administration was only able to reduce those levels of galectin-3 in HFD-MI animals.2 Myocardial ischemia in the context of obesity was also characterized by an increase in myocardial collagen content.2 Despite MCP was able to reduce collagen content in obese-MI rats, it was not able to prevent the increase in cross-linked collagen which is more resistant to degrade (Fig. 1A, B). This increase in interstitial fibrosis could also be due to an increase in its synthesis, as suggested by the rise in gene expression of collagen I (Col1a1; Fig. 1C), as well as in the profibrotic mediator Connective Tissue Growth Factor (Ctgf) (Fig. 1D). Moreover, we observed a similar but not significant trend in the gene expression of other profibrotic mediator, Transforming Growth Factor-beta (Tgf-β) (Fig. 1E). Treatment with MCP was able to prevent all these alterations in gene expression (Fig. 1D, E) as well as the increase in another ECM component such as fibronectin (Fig. 1F). The fibrosis observed in obese infarcted rats was accompanied by a reduction in both systolic and diastolic functions, which were improved in those animals receiving MCP treatment, as we previously described.2 Echocardiographic analyses revealed structural changes in HFD-MI characterized by an increase in both end-systolic diameter (ESD) and posterior wall thickness (PWT), although no major changes were observed in either interventricular septum thickness (IVST) or end-diastolic diameter (EDD). MCP treatment was able to prevent these changes (Table 1).
Effect of modified citrus pectin on cardiac fibrosis in obese infarcted rats. (A) Quantification of cross-linked collagen volume fraction; (B) representative microphotographs of cardiac sections staining with picrosirius red under polarized light (magnification 40×). Gene expression of (C) collagen I (Col1a1), (D) connective tissue growth factor (Ctgf), (E) transforming growth factor-beta (Tgf-β) and (F) fibronectin in heart of rats subjected to a sham operation (CT) and obese rats submitted to myocardial infarction treated with vehicle (HFD-MI) or with modified citrus pectin (MCP: 100mg/kg/day; HFD-MI+MCP). Bars graphs represent the mean±SEM of 7–9 animals. *p<0.05, ***p<0.001 versus CT group. †p<0.05, ††p<0.01 versus HFD-MI group.
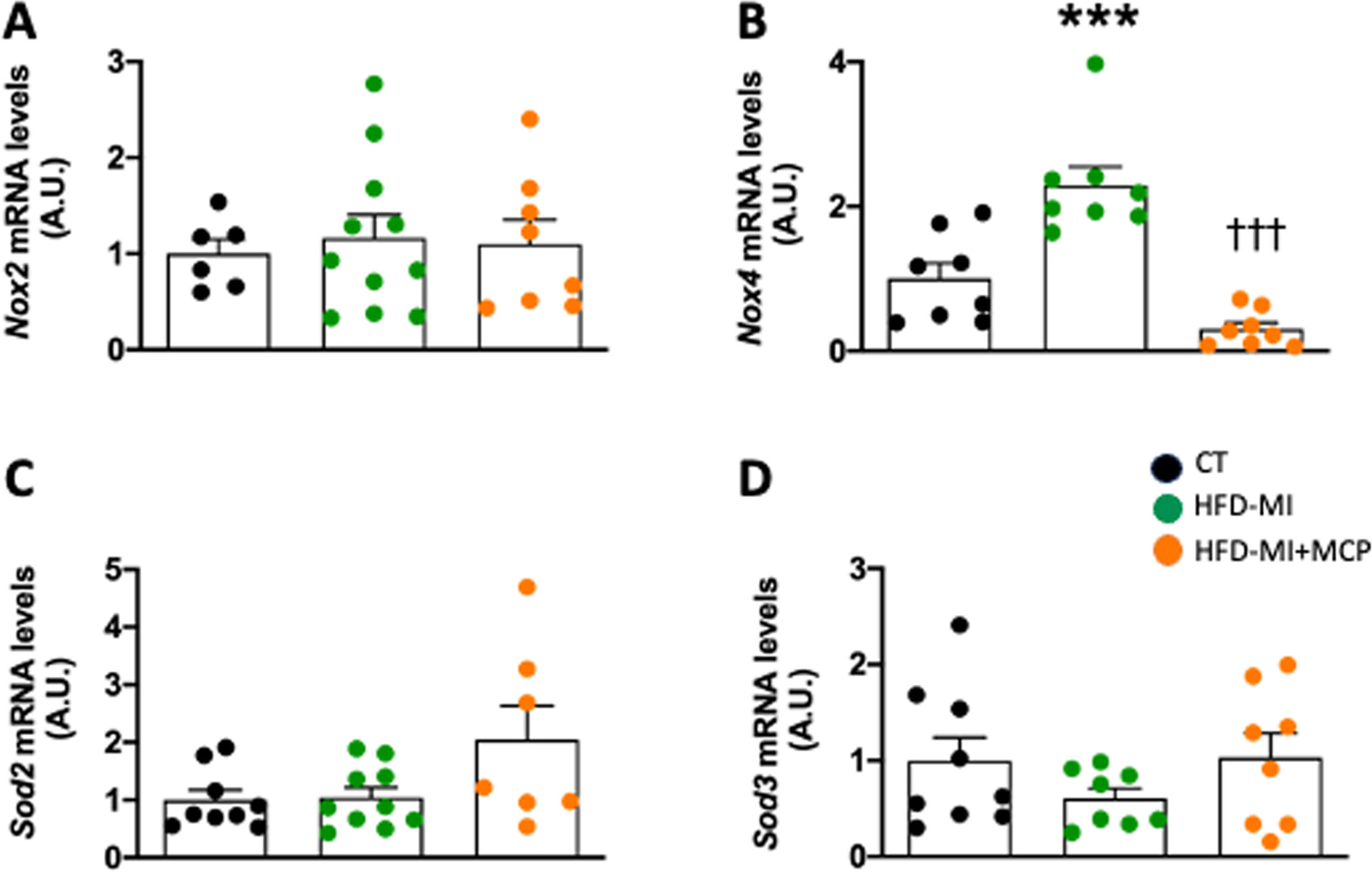
Although no changes were observed in NADPH oxidase 2 (Nox2) mRNA levels in HFD-MI group (Fig. 2A) an upregulation was observed in those of Nox4, which were reduced in MCP-treated animals (Fig. 2B). These levels were correlated with those of collagen I (r=0.5687; p=0.0037), fibronectin (r=0.5216; p=0.0183), as well as with those of Tgf-β (r=0.4985; p=0.0214) and Ctgf (r=0.8641; p<0.0001), thus supporting the interaction between oxidative stress and ECM production. No major changes were observed in the levels of two components of antioxidant defense superoxide dismutase (Sod) 2 and Sod3 (Fig. 2C, D).
Effect of modified citrus pectin on cardiac oxidative stress in obese infarcted rats. Gene expression of (A) NADPH oxidase 2 (Nox2); (B) NADPH oxidase 4 (Nox4), (C) superoxide dismutase 2 (Sod2) and (D) superoxide dismutase 3 (Sod3) in heart of rats subjected to a sham operation (CT) and obese rats submitted to myocardial infarction treated with vehicle (HFD-MI) or with modified citrus pectin (MCP: 100mg/kg/day; HFD-MI+MCP). Bars graphs represent the mean±SEM of 7–10 animals. ***p<0.001 versus CT group. †††p<0.001 versus HFD-MI group.
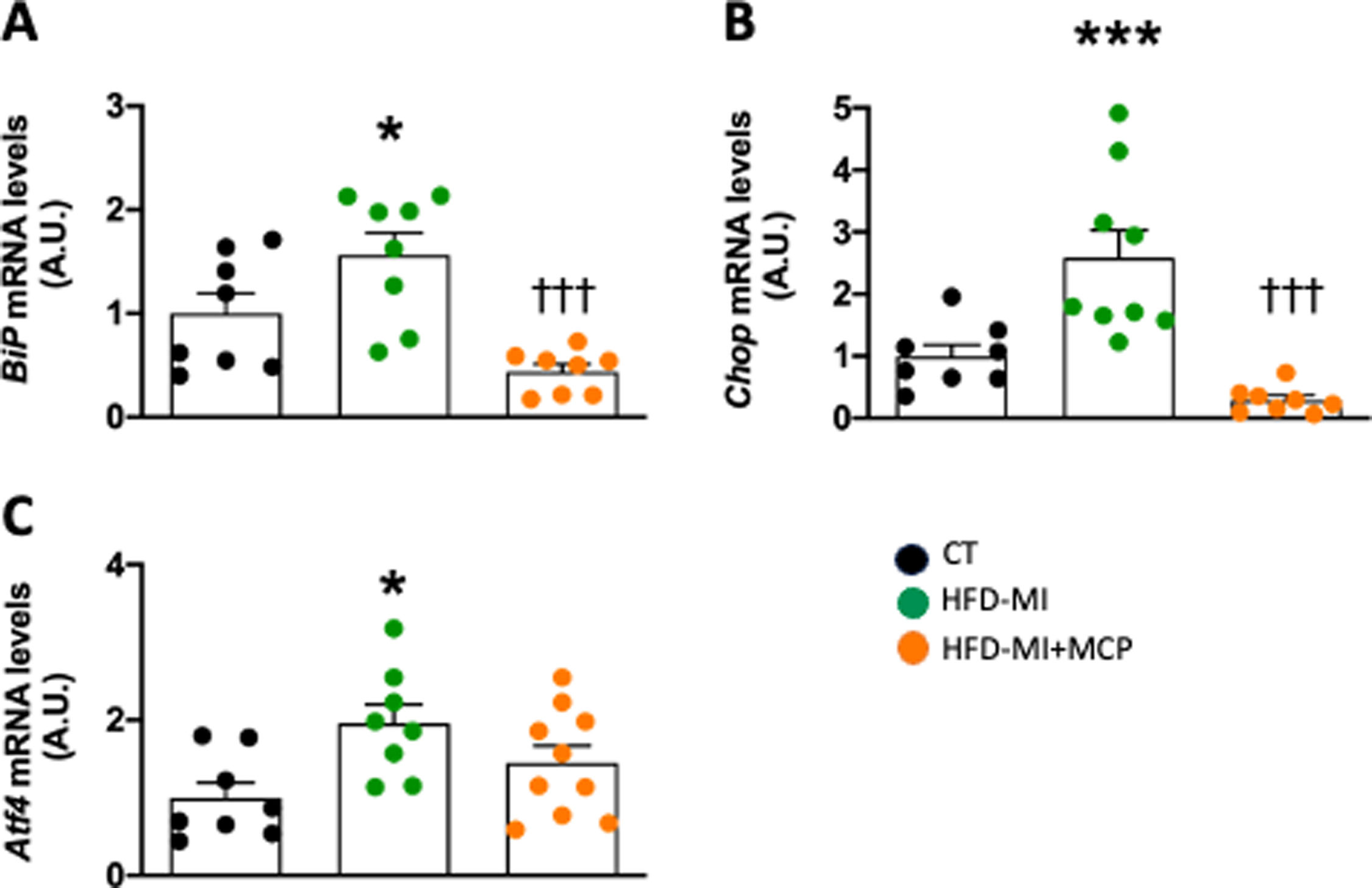
Obese infarcted rats showed an activation of endoplasmic reticulum (ER) stress, suggested by the increase in gene expression of the marker of activation binding immunoglobulin protein (BiP), as well as two down-stream mediators, CCAAT-enhancer-binding homologous protein (Chop) and activating transcription factor 4 (Atf4). These levels were reduced in animals that received MCP (Fig. 3A–C). Correlation was also found between the levels of Nox4 and with those of BiP (0.8550; p<0.0001), Chop (0.7952; p<0.0001) and Atf4 (r=0.7871; p<0.0001).
Effect of modified citrus pectin on cardiac endoplasmic reticulum stress activation in obese infarcted rats. Gene expression of (A) binding immunoglobulin protein (BiP), (B) CCAAT-enhancer-binding homologous protein (Chop) and (C) activating transcription factor 4 (Atf4) in heart of rats subjected to a sham operation (CT) and obese rats submitted to myocardial infarction treated with vehicle (HFD-MI) or with modified citrus pectin (MCP: 100mg/kg/day; HFD-MI+MCP). Bars graphs represent the mean±SEM of 7–10 animals. *p<0.05, ***p<0.001 versus CT group. †††p<0.001 versus HFD-MI group.
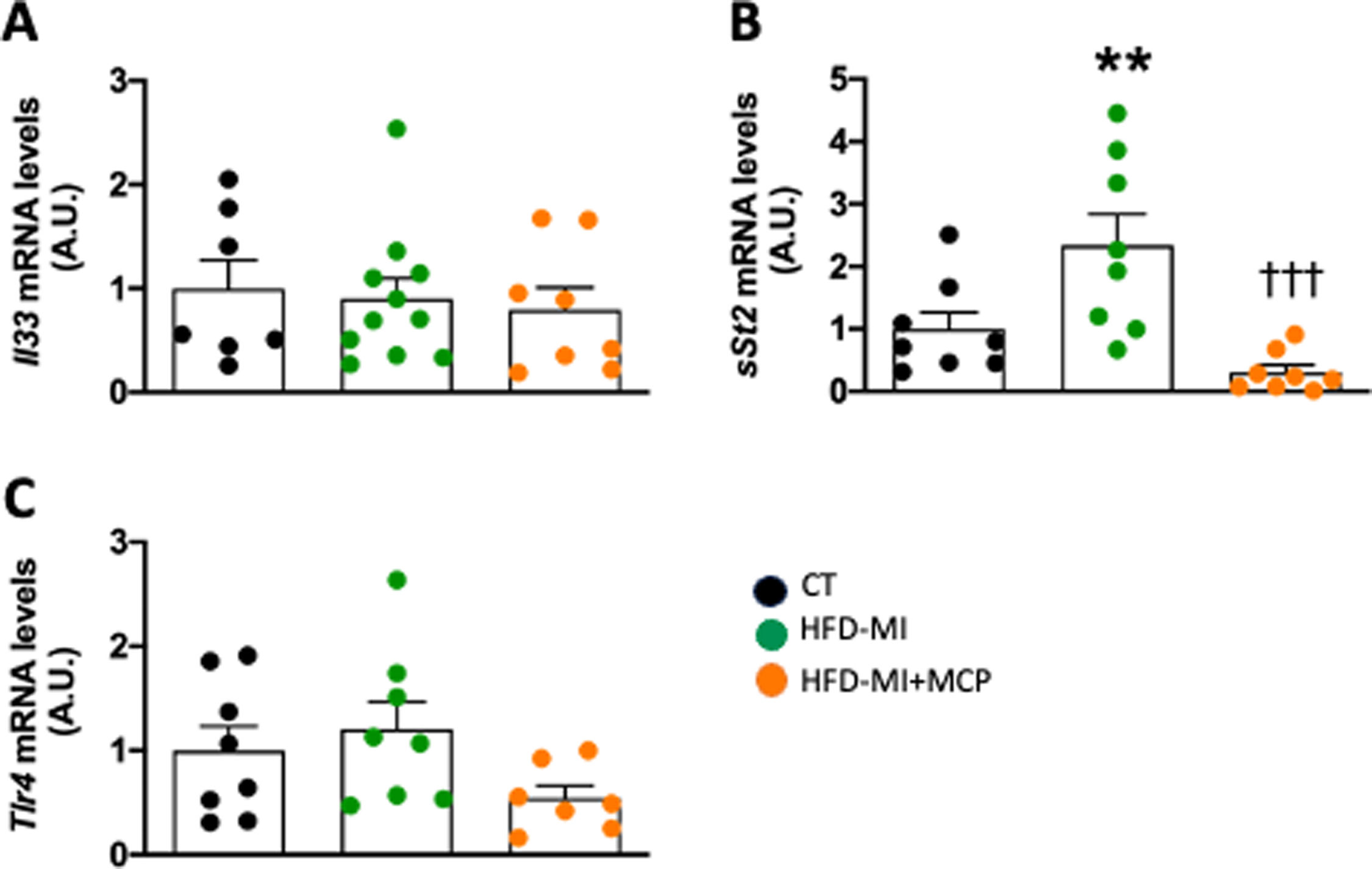
MCP was able to interact with interleukin (Il) 33/ST2 system. Despite HFD-MI animals did not show changes in Il33 mRNA levels, an upregulation in its soluble circulating receptor (sSt2) was observed (Fig. 4A, B). MCP was able to prevent the increase in sSt2 observed in obese-infarcted animals (Fig. 4B). No differences were observed in the levels of Toll-like receptor 4 (Tlr4) in any group (Fig. 4C).
Effect of modified citrus pectin on cardiac inflammation in obese infarcted rats. Gene expression of (A) Interleukin 33 (Il33), (B) soluble circulating receptor (sSt2) and Toll-like receptor 4 (Trl4) in heart of rats subjected to a sham operation (CT) and obese rats submitted to myocardial infarction treated with vehicle (HFD-MI) or with modified citrus pectin (MCP: 100mg/kg/day; HFD-MI+MCP). Bars graphs represent the mean±SEM of 7–10 animals. **p<0.01 versus CT group. †††p<0.001 versus HFD-MI group.
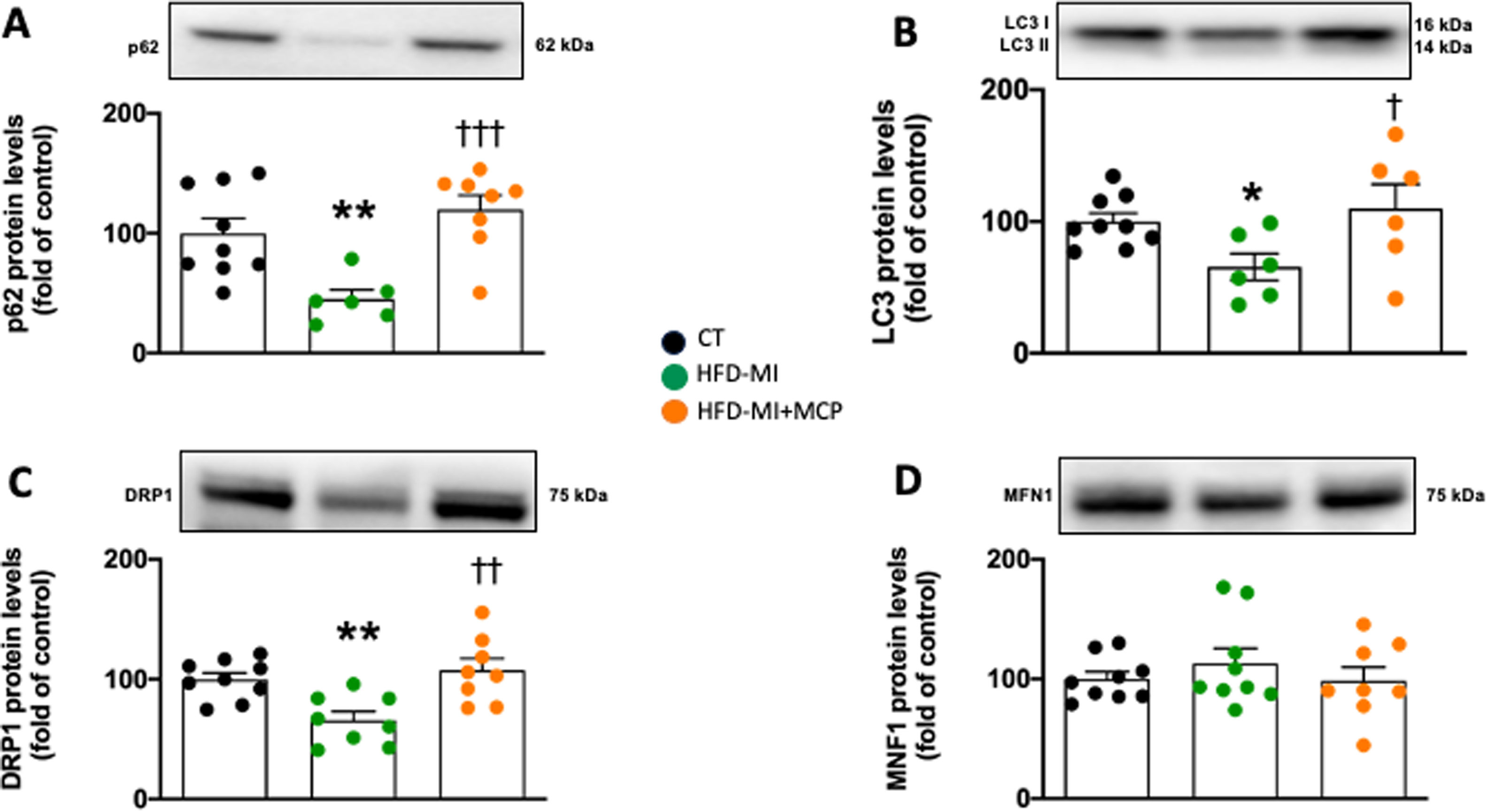
Myocardial ischemia in the context of obesity could reduce the autophagy flux, as suggested by the decreased protein levels of the markers p62 and LC3I/II ratio in HFD-MI compared with control rats (Fig. 5A, B), and which were improved in those animals that received MCP treatment (Fig. 5A, B). At mitochondrial level, obese infarcted animals showed a reduction in protein levels of DRP1 as compared with control animals, which were improved in MCP-treated animals (Fig. 5C). However, no changes in MFN1 protein levels were observed in HFD-MI independently of whether they received MCP treatment (Fig. 5D).
Effect of modified citrus pectin on cardiac mitochondrial dynamics and autophagic flux in obese infarcted rats. (A) Protein levels of p62, (B) ratio of LC3II/I protein level, (C) Levels of dynamin-1-like protein (DRP1) and (D) protein levels of mitofusin 1 (MFN1) in heart of rats subjected to a sham operation (CT) and obese rats submitted to myocardial infarction treated with vehicle (HFD-MI) or with modified citrus pectin (MCP: 100mg/kg/day; HFD-MI+MCP). Bars graphs represent the mean±SEM of 7–9 animals. *p<0.05, **p<0.01 versus CT group. †p<0.05, ††p<0.01, †††p<.001 versus MI-HFD group.
The use of nutritional supplements is widespread in hopes of enhancing physical performance but can also provide health benefits and improve some medical conditions. The present study shows that the administration of MCP was accompanied by amelioration of the cardiac consequences of myocardial ischemia in the context of obesity. These beneficial effects include a reduction in both cardiac fibrosis and oxidative and inflammatory environments. In addition, MCP treatment ameliorated the changes observed in mitochondrial fission and in autophagic markers, which could participate in the beneficial effects induced by MCP without affecting body weight or adiposity index. All these beneficial effects could involve the capacity of MCP to inhibit galectin-3 activity.
Fibrosis is common burden observed in the cardiac complications of different pathological contexts including myocardial ischemia and obesity.2,8,13 Increased accumulation of interstitial collagen leads to stiffness and alterations in the architecture of the heart with consequences for cardiac function, thus confirming our observations.2 The cardiac fibrosis observed in obese-infarcted rats seems to be an accumulation of ECM rich in fibers of collagen with a high level of cross-linking, which is more resistant to mechanical degradation and deformation. Although collagen is the most abundant cardiac ECM protein, fibronectin seems to play a role in cardiac fibrosis in the context of myocardial ischemia and obesity as suggested by the higher levels observed in HFD-MI animals in comparison to with control ones. Fibronectin is an ECM component that can influence a variety of crucial cellular processes involved in wound healing, including adhesion, growth, proliferation, migration, survival and differentiation.14 The observation that MCP treatment reduce the increase in collagen I, fibronectin and the profibrotic mediators TGF-β and CTGF13 gene levels observed in obese-infarcted rats suggests that the reduction in fibrosis by the treatment seems to be mainly consequence of ECM synthesis reduction more than changes in ECM characteristics since MCP treatment did not affect the cross-linked collagen content supporting previous observations.8,10
As expected, obese-infarcted rats showed an increase in oxidative stress, a common feature in myocardial ischemia and obesity.15,16 This oxidative environment seems to rely, at least in part, on an upregulation of Nox4, one of the main cardiac isoforms of this enzyme responsible for free radical production.17 However, no changes were observed in the gene levels of Nox2, the other main isoform. Nox4 expresses constitutive activity and can exert a protective role at cardiac level.17 However, its levels are upregulated in response to hypoxia, myocardial ischemia, and other cardiac insults.17,18 Thus, Nox4 can participate in cardiac fibrosis and hypertrophy, as it has been demonstrated in different situations including pressure overload.17–20 The present data suggest this participation in myocardial ischemia in the context of obesity since a correlation between Nox4 levels and collagen I and fibronectin were observed. The fact that Nox4 levels were also associated with those of two well-known profibrotic mediators (TGF-β and CTGF) involved in cardiac fibrosis21 further support this concept.
Nox4 is found in the membrane of ER,17 whose function is affected by both myocardial ischemia and obesity.22,23 In fact, we have reported the role of ER stress in the development of cardiac fibrosis and functional alterations in obese-infarcted rats.2 Along these lines, the data show an association between Nox4 levels and the marker of ER stress, BiP, as well as between two down-stream mediators of ER stress (CHOP and ATF4), supporting that the oxidative environment could trigger a cascade of events including ER stress that could facilitate the development of fibrosis through ECM deposition. Considering the lack of changes in the levels of two isoforms of the antioxidant defense (SOD),24,25 it could suggest that the oxidative environment that occurs in the myocardial ischemia in the context of obesity is mainly due an increase in ROS production more than a reduction in the capacity of their elimination. Our data support a role of IL33/ST2 system in the cardiac damage observed in HFD-infarcted animals as suggested by the increase in sST2 levels without changes in Il33. This is released in response to cardiac damage to promote cell survival by binding the transmembrane isoform of ST2 (ST2L).26 However, its protective effect can be silenced by increased levels of the soluble isoform of ST2 (sST2), which acts as a decoy receptor to silence IL33/ST2L signaling and trigger cardiac damage, in part, by the development of fibrosis.26,27 This role could involve triggering an inflammatory environment and aberrant fibrosis in the heart and the consequent functional alterations. In fact, there has been suggested a prognostic value of sST2 in heart failure.27,28
The cardiac remodeling that occurs after myocardial ischemia involves different processes including cell death, inflammation, synthesis, and deposition of the ECM components, resulting in changes in cardiac structure and function.29,30 Within this adaptation process autophagy plays a central role through the elimination of proteins and damaged organelles to protect myocardial cells.31 The present data suggest that MCP is able to ameliorate the alteration in autophagic flux observed in obese infarcted rats, which was accompanied by an improvement in cardiac function2 as well as a reduction in oxidative stress, inflammation and fibrosis, thus supporting the relevance of this essential cellular process for reducing heart maladaptation to myocardial ischemia in the context of obesity. It is important to mention that autophagic flux alteration has not only been reported after MI32,33 but also with obesity,34 as well as in the concomitant presence of both pathological conditions.2 Different triggering could be underlying the autophagic flux alteration observed in HFD-MI rats including ER stress. In this context, a previous study has demonstrated that the administration of an inhibitor of ER stress prevents the reduction in autophagy observed in obese-infarcted rats.2 Different mechanisms could be involved in this process including the inhibition of mTOR induced by BiP.35 On the other hand, an additional mechanism could involve an increase in the protein Atg5 by the PERK/eIF2α pathway as has been reported in other pathological contexts.36
Mitochondria are highly dynamic organelles that undergo continuous fusion and fission processes to maintain an adequate function in response to different insults. Our data support an imbalance in mitochondrial dynamics as consequence of a reduction in the fission process, as suggested by the reduction in DRP1 levels in HFD-MI animals involved in the removal of impaired elements through autophagy (mitophagy).37 This deleterious environment can contribute to mitochondrial dysfunction and the consequent cell damage. Besides its participation in mitochondrial fission, DRP1 plays an important role in mediating many aspects of mitochondrial function by stimulating mitochondrial respiration, bioenergetics and ROS signaling in adult cardiomyocytes,38 thus supporting a modulator role of DRP1 in survival and death of cardiomyocytes by affecting different mechanisms. The fact that MCP improved these changes supports a potential role of MCP on mitochondrial function.
All these cardiac beneficial effects induced by MCP in the context of myocardial ischemia and obesity could rely on its capacity to inhibit galectin-3 activity.2,7,8 In addition, a reduction in cardiac galectin-3 levels after MCP treatment has been reported in obese animals with or without MI.2,8 The key role of galectin-3 in different pathological contexts including in cardiac damage associated with obesity and myocardial infarction is nowadays well accepted.2,5,7,8 This role could involve its capacity to trigger a prooxidant and proinflammatory environment that can facilitate its profibrotic activity.7,8 This role is also supported by its prognostic value for predicting major cardiac events in different scenarios including in patients with coronary artery disease, with acute MI or heart failure.39–41 Our data not only further supported this role in a more complex situation, myocardial ischemia in the context of obesity, but also the capacity of galecin-3 in facilitating the cardiac damage observed by affecting mitochondria dynamics and autophagic flux. Protective42,43 and deleterious43,44 effects of galectin-3 on mitochondria activity have been reported in different type of cells. Our data support a deleterious effect in the heart obese infarcted rats by reducing the levels of DRP1, an essential protein in the mitochondrial fission that is able to modulate other aspects of mitochondrial function, including respiration, bioenergetics and oxidative stress signaling in cardiomyocytes.38 A variety of results regarding the impact of galectin-3 on autophagy has been also reported, since potentiation or inhibition has been found in vivo and in vitro studies in different contexts.45–47 Our data suggest that galectin-3 alters autophagic flux at cardiac level, a process essential for protecting cardiac cells during the remodeling that occurs after myocardial infarction.31
In summary, the data show the beneficial effect of the nutritional supplement MCP on the cardiac consequences associated with myocardial ischemia in the context of obesity. This role could rely on its capacity to inhibit galectin-3, a central player in the modulation of different downstream mechanisms, including inflammation, ER stress, oxidative stress, autophagy and mitochondrial function that can facilitate fibrosis and cardiac remodeling in this pathological context. Therefore, the data suggest that the consumption of MCP could help in the management of cardiac consequences in myocardial ischemia in the context of obesity. However, additional studies need to be performed to confirm whether this benefit could be translated to the clinical setting.
Authors’ contributionsEMM, VC and MLN contributed to the study conception and design. All authors performed material preparation and data collection and data analysis. EMM and VC administered the project and funding acquisition. The first draft of the manuscript was written by SJG, EMM and VC, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
FundingThis research was funded Fundación Española de Arteriosclerosis (Beca FEA 2020, NUTRICIÓN “MANUEL DE OYA). The study was also funded by Instituto de Salud Carlos III-Fondo Europeo de Desarrollo Regional (FEDER) (PI21/0431; CIBERCV). S.J.-G. and M.C.-C. were supported by a contract from Universidad Complutense de Madrid y Banco Santander (CT58/21-CT59/21 and CT15/23, respectively). B.D.-V. was supported by a grant P-FIS (FI19/00277). A.M.-G. was supported by a contract from CAM (Ayuda de empleo juvenil CT41/22/PEJ-2021-AI/BMD-22002).