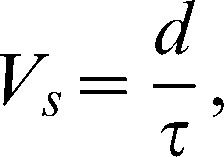To compare normal and delayed bone healing by measuring ultrasound conduction velocity across the bone callus.
METHODS:A model of transverse linear and 5 mm resection osteotomies of sheep tibiae was used. Fourteen sheep were operated on and were divided into two groups of seven according to osteotomy type. The procedure was performed on the right tibiae and the intact left tibiae were used as controls. The transverse and axial ultrasound velocities were measured at 30-day intervals for 90 days, after which the animals were killed and both the right and left tibiae were resected for in vitro biomechanical analysis.
RESULTS:Both the transverse and axial ultrasound velocities progressively increased, but the increase was smaller for the delayed union that resulted from the resection osteotomy. The mechanical resistance was higher for the normally healed tibiae that resulted from a linear osteotomy; this result closely correlated with the ultrasound velocity results. Significant differences were found for the comparisons between the intact and operated tibiae in both groups and between the groups for both the transverse and axial ultrasound velocities, but the differences were greater for the latter.
CONCLUSION:We conclude that in vivo transverse and axial ultrasound velocities provide highly precise information about the healing state of both linear and resection diaphyseal osteotomies, but the axial ultrasound velocity most likely has greater discriminatory power. This method has the potential for clinical application in humans.
Within certain values of power and intensity, ultrasound is an ionizing radiation-free physical phenomenon that is devoid of deleterious effects on living organisms, including on human beings. Ultrasonometry consists of measuring the ultrasound velocity and attenuation through any solid or liquid material, thus providing an indirect measure of anisotropy (the variation of physical properties according to direction). This capability is the basis for the use of ultrasound to measure bone mass, to quantify osteoporosis and to estimate fracture risk and this use began approximately 30 years ago (1,2). Unfortunately, trabecular alignment and bone connectivity influence the ultrasound waves that travel through bone (3). The ultrasound velocity depends on the physical properties of bone tissue, such as the elasticity, density and homogeneity (2) and positively correlates with the mechanical resistance or the rigidity of bone (4).
When obtaining measurements of a fractured bone, the fracture line, which decreases the ultrasound velocity and the formation and maturation of the bone callus, which induces a gradual increase in the ultrasound velocity, both influence the ultrasound velocity. In fact, experimental and clinical investigations have demonstrated that the ultrasound velocity steadily increases as a fractured bone heals and that the velocity slowly approaches normal values in uneventful healing (5-8); however, this behavior is not clearly demonstrated in delayed healing or in fracture nonunion.
Ultrasound velocity varies between different bones and between homonymous bones in the same subject; thus, there is no fixed value to indicate complete healing. However, the bone density and biomechanical properties of an intact homonymous contralateral bone do not change significantly during the healing period for a given fracture (6,9,10-14). Thus, the intact homonymous bone can be used for comparison; the ultrasound velocity that is measured in a region corresponding to the fracture site is considered the normal value that should be reached when the fracture is completely healed, either in experimental investigations with animals or in clinical situations in humans.
Bone ultrasonometry can be performed using underwater or direct-contact techniques and using either the transverse or axial transmission modality. From a clinical standpoint, the direct contact technique, which involves the interposition of a coupling gel, is the most applicable to clinical situations; the transverse transmission modality is applicable to the evaluation of both superficial and deep bones, whereas the axial modality seems to be adequate only for superficial bones (10,13,15,16).
Until now, the ultrasound velocity through a healing anomaly (delayed union, nonunion) has not been well studied despite the potential clinical use of the method as an ancillary method of diagnosing a healing anomaly. Because the ultrasound velocity increases to normal values as healing occurs in a normal healing situation, we hypothesized that the velocity would remain well below normal values for a healing anomaly. Thus, the objective of the present investigation was to measure the in vivo direct contact transverse and axial ultrasound velocities to evaluate the delayed healing that results from a transverse resection osteotomy of the midshaft of sheep tibiae by comparing these velocities with those for the uneventful healing that results from a simple transverse osteotomy. Using the same experimental design, both types of osteotomy were fixed with a semi-flexible external fixator, which enhances the production of a bulky bone callus.
MATERIAL AND METHODSThis experiment was approved by the Ethics Committee on the Experimental Use of Animals of the authors' institution. Fourteen young adult (∼15 months of age) sheep of the Santa Ignez breed were divided into two groups of seven animals each according to the type of procedure. The operations were performed on the right tibiae (henceforth referred to as operated) and the left tibiae were untouched (henceforth referred to as intact). The normal control group consisted of the fourteen intact tibiae, which were subjected to the same in vivo and postmortem studies.
Operative procedureBecause the animals were not intubated, they were fasted for 24 hours before the operation to avoid intraoperative problems with vomiting and aspiration. The procedure was performed under general intravenous anesthesia (jugular vein; ketamine, 3 mg/kg; xylazine, 0.1 mg/kg; acepromazine, 0.1 mg/kg; tramadol, 2 mg/kg) with continuous administration of plain oxygen through a nostril. The operation began with the installation of a semi-flexible external fixator on the anteromedial aspect of the right tibia, into which four Shanz-type self-tapping threaded 4 mm-diameter pins were perpendicularly introduced at a distance of 5 cm from one another. First, holes 3 mm in diameter were diametrically perforated through 1.5×1.5 skin cross incisions with the assistance of a jig and down to the opposite cortex. Each pin was then manually introduced, the fixator's semi-flexible connecting bar was installed and, with all bolts tightened, the tibial shaft was exposed through a 3 to 4 cm-long longitudinal skin incision between the two central pins; the periosteum being was incised along the same line. A subperiosteal transverse linear osteotomy was performed in the Group 1 tibiae and a 5 mm-thick resection transverse osteotomy was performed in the Group 2 tibiae. Both osteotomies were performed at the midpoint between the medial condyle and the medial malleolus with an oscillatory saw with a 0.8 mm-thick blade. The periosteum and skin were closed with separate stitches of absorbable suture (4/0 Monocryl®, Ethicon, São José dos Campos, Brazil) and an occlusive dressing was applied to protect both the incision and the entry points of the pins. The animals were allowed to walk freely immediately after recovering from the anesthesia.
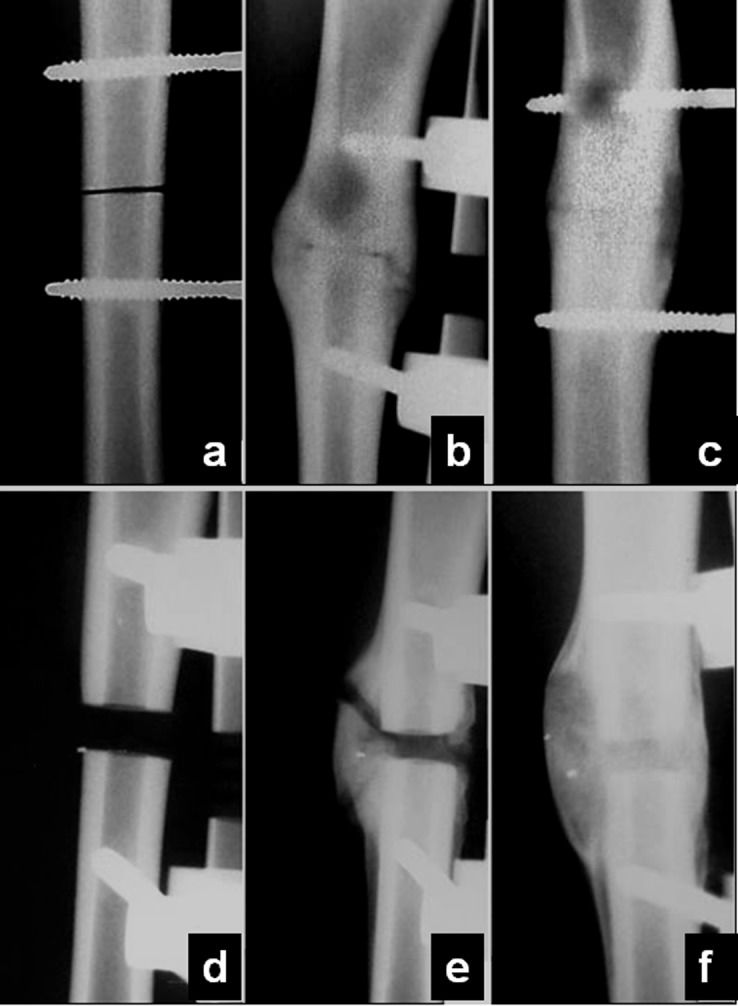
Postoperative managementThe animals were given intramuscular prophylactic antibiotics (crystalline penicillin, 40000 IU/kg, a single dose at the end of the operative procedure) and analgesics (ketoprofen, 2 mg/kg TID, for 5 d). Control radiographs were taken immediately postoperatively and at 30-d intervals (on the 30th, 60th and 90th postoperative days) to check the fragment position and the fixator stability (Figure1). All animals were killed on the 90th postoperative day with an intravenous injection of excess general anesthetic (thiopental) after the radiographic and ultrasonometric evaluations were performed. Both the right and left tibiae were resected and were carefully stripped of all soft tissues for in vitro biomechanical analysis.
Ultrasonometric analysisThe ultrasound velocity was measured on the 30th, 60th and 90th postoperative days using an ultrasound generator-receiver-amplifier source (Biotecnosis do Brasil Ltda., Model US01, Ribeirão Preto, São Paulo, Brazil, www.biotecnosis.com) equipped with two unfocused ultrasound transducers (2 mm-thick PZT-5 disc, 12 mm in diameter, 1 MHz frequency), one for emission and the other for reception. The ultrasound source was able to generate high-power (up to 300 V), narrow (1 µs), well-defined ultrasonic pulses. The source was linked to a computer loaded with software capable of automatically calculating the ultrasound velocity based on the emitted ultrasound signal and the time of flight of the first-arriving signal (FAS), which was identified on the digital storage oscilloscope (Agilent Technologies, Inc., model DSO3062A, Shangai, China). The equipment was calibrated after every five measurements using a 20 mm-thick Teflon disc to check for regularity and accuracy because the ultrasound velocity through this disc was known to be constant (1274 m/sec at 35°C, 0.3% variation).
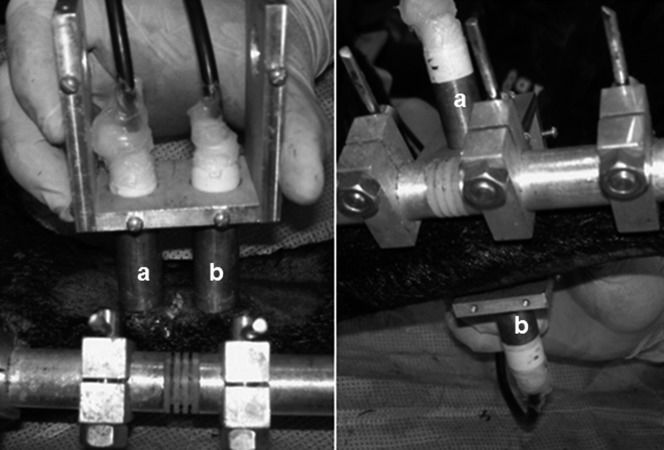
Transcutaneous direct contact transverse and axial ultrasound velocity measurements were conducted for both the operated and intact tibiae. The fourteen intact tibiae were used as a single control group for Groups 1 and 2. The transducers were mounted facing each other onto a U-shaped accessory and were aligned with each other along the central axis for the transverse modality, thus positioning one on each side of the leg. For the axial modality, the transducers were mounted parallel to each other for tandem positioning with one above (emitting) the osteotomy site and the other below (receiving) (Figure2). The transducers were carefully attached to the skin without squeezing it and were aligned as precisely as possible with the transverse tibial axis for the transverse modality and with the longitudinal tibial axis for the axial modality. Topographic anatomical parameters (tibial crest, sagittal diameter) and the two central Shanz pins were used for guidance, and abundant ultrasonic coupling gel was used to ensure close contact with the skin. The distance between the transducers' active surfaces (corresponding to the coronal diameter at the osteotomy site) was taken as the reference distance for calculating the transverse ultrasound velocity; this distance varied for the right and left legs, from animal to animal and from period to period. The distance between the transducers' centers was the reference distance for the calculation of the axial ultrasound velocity and was fixed for all situations (23.5 mm) because of the U-shaped accessory.
The time elapsed between the emitted ultrasound signal and the first-arriving signal (FAS) was automatically sent to the computer software, but the variable distance, which consisted of the total leg thickness for the transverse ultrasound velocity and the distance between the transducers' centers for the axial ultrasound velocity, was entered manually. Five consecutive ultrasound velocity measurements were obtained using each modality and an average value was calculated and used for the analyses; the highest and the lowest values were discarded to ensure homogeneity. Both the transverse and axial ultrasound velocities were calculated using the following equation:
where Vs = velocity through the leg; d = distance (frontal diameter of the specimen for the transverse velocity and distance between the centers of the transducers for the axial velocity); and τ = time for specimen.Angular deformationThe angular deformation by manipulation was measured at all postoperative time points, to mimic the clinical examination of the healing of a fracture. The fixator's connecting bar was temporarily removed immediately after each ultrasonometric analysis to check for fracture stability, by measuring the total deformation imposed by a single angular motion on the coronal plane, beginning from the neutral position toward the medial and then toward the lateral side of the leg, without excessively forcing it in order to avoid callus disruption. The total angular deformation was measured (○) with a clinical goniometer aligned with the anterior tibial crest and later compared with ultrasonometric and biomechanical resistance data.
Biomechanical analysisBoth the operated and intact tibiae of Groups 1 and 2 were subjected to a destructive three-point maximum load bending assay using a universal testing machine (EMIC®, model DL10000, São José dos Pinhais, Paraná, Brazil) with a high-precision 1000 kgf load cell. During these assays, the tibiae were positioned horizontally and the proximal and distal ends were supported on rounded wedge-shaped stands separated from each other by 130 mm and from the osteotomy line (located as described above) by 65 mm. The load was applied vertically on the sagittal plane of the tibia and was placed as precisely as possible at the osteotomy line using a rounded wedge-shaped accessory to avoid penetration of the bone tissue (Figure3). The assay began with the application of a 100 N pre-load for 60 seconds for system accommodation; the actual load was then continuously applied at a rate of 1 mm/min until failure, usually due to a fracture at the osteotomy site, which corresponded to the maximum load supported by the bone. The maximum load value was recorded and was later compared with the ultrasonometric parameters.
Statistical analysisThe transverse and axial ultrasound velocity data were compared between the operated (Groups 1 and 2) and intact tibiae, within each postoperative period and between periods (30, 60 and 90 days) using a mixed effects linear regression model adjusted by the Proc Mixed procedure of SAS v.9 software at the 1% level of significance (p≤0.01). An analysis of residues was performed to check the assumptions (normal distribution of residues with a mean of zero and variance of Σ2) and the logarithmic transformation was found to be adequate to satisfy this demand in some cases (17,18,19). The raw averages were also compared for each situation.
RESULTSBoth the anesthetic and the operative procedures were well tolerated by the animals. The surgical wounds healed uneventfully in all of the animals and they appeared to resume total weight-bearing on the operated leg and the use of a normal gait at approximately the 7th postoperative day.
Leg thicknessThe leg thickness, measured as the coronal transverse diameter of the leg at the osteotomy site, was consistently thinner for the Group 1 tibiae (linear osteotomy) than for the Group 2 tibiae (resection osteotomy) in all periods. The fourteen intact tibiae were used as a control for both groups. The thickness slowly decreased over time in both groups but remained wider for the Group 2 tibiae on day 90 than for the Group 1 tibiae on day 30 because of the formation of a bulky callus in Group 2.
Angular deformationAs expected, the angular deformation progressively decreased over time; it was <3° on average for the Group 1 tibiae and 7° on average for the Group 2 tibiae on day 30. The deformation decreased to 0° on day 60 and remained at that level on day 90 for Group 1; therefore, from a clinical standpoint, the Group 1 tibiae were effectively healed on day 60. The Group 2 tibiae were still partially mobile on day 60 (3°), but on day 90, clear mobility was only observed in two cases in which the deformation was approximately 3° for each; thus, the deformation was less than 1° on average for the whole group (including the two tibiae with deformations of approximately 3°).
Radiographic appearanceThe conventional radiographic control showed very little callus formation around the osteotomy site in both Groups 1 and 2 on day 30. The volume of the callus increased significantly on day 60 in both groups, although the Group 2 tibiae continued to show a wide osteotomy line. On day 90, the callus was almost completely remodeled in Group 1, whereas a quite bulky callus continued to be visible around the resection osteotomy in Group 2 (Figure4).
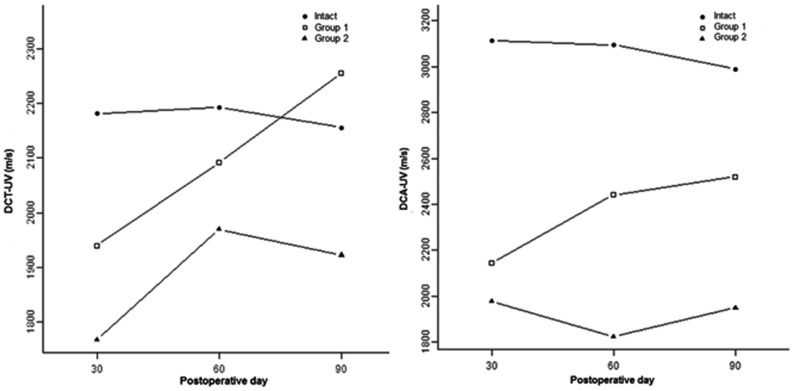
Transverse ultrasound velocityThe transverse ultrasound velocity data are shown in Table1. The average transverse ultrasound velocity for the intact tibiae was 2181 m/s, 2193 m/s and 2156 m/s on days 30, 60 and 90, respectively, with no significant difference between the periods (p = 0.83 for days 30×60; p = 0.64 for days 30×90; and p = 0.50 for days 60×90). For the operated tibiae in Group 1 (linear osteotomy), the average transverse velocity was 1940 m/s, 2092 m/s and 2256 m/s at the same postoperative time points as above, with a significant difference (p≤0.01) between days 30 and 90 but not between days 30 and 60 (p = 0.04) or days 60 and 90 (p = 0.03). For the operated tibiae in Group 2 (resection osteotomy), the average transverse velocity was 1768 m/s on day 30, 1969 m/s on day 60 and 1922 m/s on day 90, with a significant difference (p≤0.01) between days 30 and 60 but not between days 30 and 90 (p = 0.03) and days 60 and 90 (p = 0.53). The differences were significant for the comparison between the intact and operated tibiae in Group 1 on day 30 (p≤0.01), but not on day 60 (p = 0.07) or day 90 (p = 0.25); the differences were also significant for all comparisons (p≤0.01) between the intact and operated tibiae in Group 2 and for the comparison between the operated tibiae of Groups 1 and 2 on day 90 (p≤0.01), but not on days 30 (p = 0.09) and 60 (p = 0.30).
-Transverse ultrasound velocity (m/s) for the intact and operated tibiae according to group and period (range, average and SD).
| Period (days) | Intact | Group 1 | Group 2 |
|---|---|---|---|
| 30 | 1963 - 2578 | 1883 - 2018 | 1615 - 1878 |
| 2181±199 | 1940±45 | 1768±79 | |
| 60 | 1955 - 2583 | 1904 - 2276 | 1718 - 2376 |
| 2193±191 | 2092±123 | 1969±216 | |
| 90 | 2000 - 2528 | 2027 - 2499 | 1805 - 2266 |
| 2156±169 | 2256±164 | 1922±163 |
The axial ultrasound velocity data are shown in Table2. The average axial ultrasound velocity for the intact tibiae was 3112 m/s, 3094 m/s and 2989 m/s on days 30, 60 and 90, respectively, with no significant difference between periods (p = 0.80 for days 30×60; p = 0.08 for days 30×90; and p = 0.14 for days 60×90). For the operated tibiae in Group 1 (linear osteotomy), the average axial velocity was 2144 m/s, 2441 m/s and 2520 m/s at the same postoperative time points as above, with a significant difference (p≤0.01) between days 30 and 60 and between days 30 and 90 but not between days 60 and 90 (p = 0.43). For the operated tibiae in Group 2 (resection osteotomy), the average axial velocity was 1976 m/s on day 30, 1824 m/s on day 60 and 1949 m/s on day 90, with no significant difference for any comparison (p = 0.13 for days 30×60; p = 0.78 for days 30×90; and p = 0.21 for days 60×90). The differences were significant for all comparisons (p≤0.01) between the intact and operated tibiae in Group 1, for all comparisons (p≤0.01) between the intact and operated tibiae in Group 2 and for all comparisons (p≤0.01) between the operated tibiae in Groups 1 and 2 on days 30, 60 and 90.
-Axial ultrasound velocity (m/s) for the intact and operated tibiae according to group and period (range, average and SD).
| Period (days) | Intact | Group 1 | Group 2 |
|---|---|---|---|
| 30 | 2676 - 3638 | 1934 - 2421 | 1671 - 2109 |
| 3112±288 | 2144±187 | 1976±143 | |
| 60 | 2701 - 3649 | 2096 - 2893 | 1623 - 2045 |
| 3094±280 | 2441±345 | 1824±147 | |
| 90 | 2616 - 3650 | 2059 - 2822 | 1642 - 2143 |
| 2989±287 | 2520±279 | 1949±178 |
In Group 1, the average maximum load for the operated tibiae was 1169 N, which corresponds to ∼87% of the average value of 1340 N obtained for the 14 intact tibiae. In Group 2, the average maximum load for the operated tibiae was 1070 N, which corresponds to 80% of the average value of 1338 N obtained for the 14 intact tibiae. Although the differences were evident, they were not statistically significant between Groups 1 and 2 (p = 0.60) or between the intact and operated tibiae of both Groups 1 (p = 0.09) and 2 (p = 0.04).
DISCUSSIONThe healing time of a fracture depends on various local and systemic factors, but any closed fracture can heal within three months from its occurrence in both animals and humans (20,21). If a fracture does not heal within three months, the situation is called delayed union; if it does not heal within six months, it is referred to as nonunion. Both delayed unions and nonunions are considered healing anomalies that require special attention because a delayed union can still heal spontaneously with time, but a nonunion will most likely never heal without additional treatment. Early diagnosis of both conditions is very important to allow for the administration of an appropriate treatment as early as possible, which will reduce the financial and social costs. Ideally, a healing anomaly should be diagnosed and treated during the delayed union phase to prevent progression to a nonunion, for which the treatment is far more difficult and is prone to further complications.
Diagnosing a delayed union is highly subjective and is not always easy because it depends on the interpretation of the radiographic appearance and image by the surgeon or the radiologist and the presence of metal implants can increase the difficulty of making this interpretation. A computer-aided tomography scan could be used for diagnostic purposes, but these involve the use of high doses of ionizing radiation and likewise suffer from the influence of metal implants; thus, these scans produce a variety of artifacts. Similar problems occur with magnetic resonance imaging, which is essentially useless in these cases. Ultrasonometry, which is virtually devoid of deleterious effects on biological tissues, appears to be a good alternative because it is a relatively economical, radiation-free physical technique that can be used to discriminate the healing status of a fracture, as demonstrated by previous clinical and experimental investigations (1,2,5,6,10,14,15,22). Ultrasonometry is based on the fact that as an ultrasound wave propagates through a fractured bone, its velocity slowly increases to finally reach nearly normal values as healing and remodeling occur; the fractured bone rebuilds its lamellar structure and calcifies, thus restoring bone anisotropy. However, the in vivo ultrasonometric behavior of a delayed union compared with that of an uneventful union has not been properly determined and performing this comparison was the specific purpose of the present investigation, which used sheep tibiae as an in vivo experimental model (14,15,22).
This investigation was designed to compare delayed healing with uneventful healing; these processes were measured in separate animal groups using linear osteotomy in the first group and resection osteotomy in the second. Similarly to previous investigations, a semi-flexible external fixator was used to stabilize both the linear (Group 1) and resection (Group 2) osteotomies; this method was quite effective at generating the expected results. In fact, the combination of the external fixator with the linear osteotomy was sufficiently rigid to provide quick uneventful healing via a small, compact extra- and intra-periosteal callus, while its combination with the resection osteotomy was sufficiently flexible to provide healing via a bulky, less dense, predominantly periosteal immature callus, which was still undergoing remodeling at the end of the third postoperative month. The immaturity of the bulky callus in Group 2 was revealed in the angular motion that occurred at the osteotomy site during the second postoperative month, in contrast to the complete immobility present in Group 1 at the same time point. Furthermore, biomechanical studies performed on the 90th postoperative day showed lower mechanical resistance, which was another indication of the immature bulky callus in Group 2.
For the ultrasonometry, the ultrasound velocity was chosen as the analysis parameter because it is a fundamental property of the acoustic propagation in tissues; this property is also easier to calculate (22,23,24,25) and is more reliable than the broadband ultrasound attenuation, the other parameter that is often used to evaluate bone mass and osteoporosis (26). The ultrasound velocity through bone negatively correlates with the ultrasound attenuation, meaning that the attenuation decreases as the velocity increases and vice versa, as demonstrated in previous investigations (14). According to a recent publication, in vivo ultrasound velocity measurements are subject to the problem of soft tissue interposition between the ultrasound transducers and the bone itself; in fact, animal legs are multi-layered structures, with considerable differences in acoustic impedance between the bone and the surrounding soft tissues (approximately 80% less). Thus, the ultrasound energy decreases with an increase in the thickness of the soft tissues, as shown by the differences in the behavior of the first- (FAS) and second-arriving signals (SAS). This difference requires proper treatment to prevent a decrease in the accuracy of the ultrasound velocity measurement (27). Although reliable, the data above were obtained in a laboratory bench investigation using soft-tissue-bone phantoms made of acrylic and silicone rubber plates and they most likely require further investigation and confirmation in a real situation using animal models and/or humans. Therefore, we decided to not consider that problem at this stage; furthermore, the present investigation had already been completed when the above-mentioned paper was published.
According to the preoperative planning, the ultrasound velocity was measured using the transverse and axial modalities because they are both applicable to the animal model used and both seem relevant to clinical applications in humans. The results were consistent with the hypothesis that the ultrasound velocity increases as healing occurs for both linear and resection osteotomies, but less so for the latter. In fact, compared with the values measured for the intact tibiae, the transverse ultrasound velocity was approximately 89%, 96% and 104% of the normal value in Group 1 and 81%, 89% and 88% of the normal value in Group 2 at the end of the first, second and third postoperative months, respectively. The axial ultrasound velocity was approximately 69%, 80% and 82% of the normal value in Group 1 and 64%, 60% and 64% of the normal value in Group 2 at the end of the first, second and third postoperative months, respectively. Therefore, both the transverse and axial ultrasound velocities appear to have increased more steadily in Group 1 than in Group 2, and the values clearly tended to remain stable in Group 2 during the second and third postoperative months. These results do not indicate a lack of healing in Group 2; instead, the healing occurred in a much slower fashion and the ultrasonometry could effectively detect such a difference in the behavior between the groups. Additionally, it is possible to divide the ultrasound wave transmission in Group 2 into different stages; the waves traveled more rapidly through the very young and partially liquid callus on day 30, but they traveled more slowly through the already fibrous callus on day 60 and the wave velocity recovered on day 90 when the callus underwent calcification. Furthermore, in the axial transmission mode, the waves tend to travel along the cortical surface instead of through the entire cortical thickness, depending on the relationship between the wavelength and the cortical thickness, as noted above. However, on day 60, the callus was still too fibrous and its acoustic impedance was too high, thus decreasing the ultrasound velocity. As soon as the callus calcified and became more solid, the acoustic impedance decreased and the velocity increased, which occurred on day 90.
The ultrasound velocity values were consistently higher for the axial measurement modality than for the transverse modality for both the intact and operated tibiae; this finding is consistent with previous observations that axial waves travel along a superficial path for the first and through the entire cortical thickness for the second, depending at least in part on the relationship between the cortical bone thickness and the ultrasound wavelength. In fact, when the wavelength is narrower than the cortical thickness, the waves propagate through the subperiosteal layer of the entry cortex (so-called superficial propagation); these waves are therefore faster than those with longer wavelengths, which propagate through the entire cortical thickness (full-thickness propagation) and are thus slower. For the 1 MHz frequency used here, the wavelength (the ultrasound velocity divided by the frequency) varied between an estimated 2 and 3 mm, whereas the total thickness of the cortex and callus together was much greater than those values in both groups; thus, these parameters indicate that the ultrasound wave propagation most likely occurred as described above.
Statistically, the axial ultrasound velocity may have been more discriminating than the transverse ultrasound velocity because the differences were significant (p≤0.01) for all comparisons, including the comparisons between the intact and operated tibiae within each group and the comparisons between the operated tibiae in both groups. However, the fact that transverse ultrasound velocity was not significantly different between the intact and operated tibiae in Group 1 during the second (p = 0.07) and third (p = 0.25) postoperative months most likely means that the remodeling was so advanced in this group that the internal structure of the operated bones was already able to conduct the transverse ultrasound waves in a nearly normal manner. In contrast, the significant difference between the intact and operated tibiae in Group 2 at all time points, particularly on day 90, most likely indicates that the massive callus that formed around the resection osteotomy was still too immature to conduct the ultrasound waves properly.
The in vitro mechanical resistance figures were clearly higher for the operated tibiae of Group 1 (87% of the normal value) than for those of Group 2 (80% of the normal value), although the difference was not significant. This difference occurred despite the bulkier periosteal callus observed in Group 2, confirming that the healing was more complete for linear osteotomies than for resection osteotomies on day 90.
In conclusion, in vivo ultrasonometric diagnosis of the healing status of experimental fractures, for both normal and anomalous healing, is feasible and measurement of either the transverse or axial ultrasound velocity can be useful for this purpose. However, the axial ultrasound velocity appears to have greater discriminatory power compared with the transverse velocity, most likely because of its different wave propagation mode.
ACKNOWLEDGMENTSThe authors acknowledge São Paulo State Research Support Foundation (FAPESP, Fundação de Amparo à Pesquisa do Estado de São Paulo) for financial support (Grants 2007/56422-0 and 2008/55342-5).
AUTHOR CONTRIBUTIONSBarbieri G was responsible for the experimental design, surgical and evaluation procedures, preliminary data interpretation and manuscript preparation. Barbieri CH was responsible for the experimental design, financial support (FAPESP - Fundação de Amparo a Pesquisa do Estado de São Paulo grant), equipment provision, final data interpretation and English manuscript preparation.
No potential conflict of interest was reported.