To evaluate the effects of three different target-controlled remifentanil infusion rates during target-controlled propofol infusion on hemodynamic parameters, pain, sedation, and recovery score during oocyte retrieval.
METHODS:Sixty-nine women were scheduled for oocyte retrieval. Target-controlled propofol infusion at an effect-site concentration of 1.5 μg/mL was instituted. The patients were randomly allocated to receive remifentanil at an effect-site concentration of either 1.5 (group I, n = 23), 2 (group II, n = 23) or 2.5 ng/mL (group III, n = 23). Hemodynamic variables, sedation, pain, the Aldrete recovery score, and side effects were recorded.
RESULTS:Hemodynamic variables, sedation and pain scores and the number of patients with the maximum Aldrete recovery score 10 min after the procedure were comparable among the groups. The number of patients in group III with the maximum Aldrete recovery score 5 min after the procedure was significantly lower than that in groups I and II. One patient in group II and one patient in group III suffered from nausea.
CONCLUSION:Similar pain-free conscious sedation conditions without significant changes in hemodynamic parameters were provided by all three protocols. However, target controlled infusion of remifentanil at 1.5 or 2 ng/mL proved superior at providing early recovery compared to 2.5 ng/mL.
Transvaginal ultrasound-guided oocyte retrieval is a relatively painful assisted reproductive technology procedure that is performed on an outpatient basis. To date, both general and regional anesthetics, including paracervicals, spinals and epidurals, have been used, and various methods of conscious sedation and analgesia have been attempted.1,2 Pain during oocyte retrieval is caused by a puncture of the vaginal skin and the ovarian capsule, aspiration of the ovary and manipulation of the ovary. Therefore, during sedation and analgesia, the role of the anesthesiologist is, primarily, to provide adequate pain relief to keep the patient immobilized during critical times and, secondarily, to provide sedation for anxious patients. Otherwise, bleeding from the puncture site and into the abdomen after oocyte retrieval can lead to hospital admission.1
Whenever favorable analgesia with sedation and rapid recovery are desired, propofol and remifentanil are the agents of choice in ambulatory settings due to their pharmacokinetic profile.3 In contrast, target-controlled infusion (TCI) is a popular system that maintains a particular target plasma drug concentration using standard pharmacokinetic equations.4,5 Although TCI has been used in many different kinds of outpatient procedures,6-10 few studies of its use during oocyte retrieval have yet been reported.11-13 To our knowledge, the dose-effect relationship of TCI of remifentanil and propofol in spontaneously breathing women scheduled for oocyte retrieval has not been investigated. Therefore, we aimed to evaluate the effects of three different TCI protocols (including remifentanil and propofol) on hemodynamic parameters, pain, sedation, the recovery score, and side effects in patients undergoing oocyte retrieval in a randomized prospective study.
METHODSAfter obtaining approval from the ethics committee and written informed consent from each patient, 69 unpremedicated women who had an American Society of Anesthesiologists physical status class I or II, were between 21 and 45 years old, and were scheduled for oocyte retrieval were enrolled. Before starting the procedure, the patients were told to evaluate their pain according to a 10-point numerical rating scale, where 0 corresponded to “no pain,” and 10 corresponded to the “worst possible pain.” Scores between 0 and 3 were accepted as relatively pain-free. Upon arrival to the anesthesia suite, intravenous infusion of Ringer's lactate (5 mL/kg/h) solution was started. Heart rate, non-invasive blood pressure and peripheral oxygen saturation (SpO2) were continuously monitored and followed every minute for 5 minutes after induction and then every 5 minutes until the end of the procedure. These parameters were recorded prior to drug administration at baseline (control), at the onset of the procedure (start), at the time of puncture and aspiration of the oocytes from each ovary (first and second puncture) and at the end of the procedure. All of the patients received 4 L/min of oxygen via a face mask.
Sedation levels were evaluated using a 5-point scale (1, fully awake and oriented; 2, drowsy; 3, eyes closed, responds promptly to verbal commands; 4, eyes closed, only aroused upon mild physical stimulation; 5, eyes closed, not aroused upon mild physical stimulation), as described by Hong et al.12 Sedation scores ≥3 were the target throughout the procedure. Whenever a patient needed a TCI rate adjustment (increase or decrease) for remifentanil, the analgesia level (according to the numerical rating scale) and the sedation score were recorded every 5 minutes until the end of the procedure, particularly at the times when the ovarian capsules were aspirated from each ovary. According to the preference of the gynecologists for standardized procedures, the right ovarian puncture (called the first puncture) was followed by the left ovarian puncture (called the second puncture) in all of the patients.
Propofol and remifentanil were administered with a TCI device (Orchestra Base Primea®, Fresenius Kabi, France), which enables the drug concentration in the blood, in the plasma (Cp) and at the effect site (Ce) to be continuously controlled.14 The device is a system that allows untagged syringes to be used with generic, low-cost propofol. The pharmacokinetic model described by Schnider et al.5 uses age as a co-variate to improve the accuracy of the model. Because the model has a smaller-volume central compartment and equilibrates more quickly with the effect site,5 our patients received TCI propofol driven by the Schnider model with effect-site control. Meanwhile, the infusion rate of TCI remifentanil was controlled by Minto's pharmacokinetic model incorporated into software that was previously used specifically for remifentanil.4,7 Syringes containing 1% propofol (10 mg/mL) and remifentanil (50 μg/mL) were simultaneously loaded onto the device and connected to the patient's intravenous catheter using a three-way stopcock. The patients were randomly allocated into 3 groups using written and enclosed computer-generated group numbers corresponding to the 3 different protocols: group I (n = 23), TCI remifentanil at Ce 1.5 ng/mL; group II (n = 23), 2 ng/mL; group III (n = 23), 2.5 ng/mL. A constant rate of TCI propofol at Ce 1.5 μg/mL at one of the three remifentanil rates was started, depending on the group assignment. The remifentanil rate was accordingly adjusted by incremental increases or decreases of 0.5 μg/mL if the numerical rating scale was greater than 3 or if the SpO2 was <95%. The propofol and remifentanil infusions were discontinued after the aspiration of the last follicle from the ovary. The total amounts of remifentanil and propofol that were used were then checked and noted. The duration of both the anesthesia and the procedure were also recorded.
In the absence of bleeding from the puncture sites, the patients were transferred to the recovery room. The Aldrete recovery score, also known as the post-anesthesia recovery score, was determined 5 and 10 minutes after the completion of the procedure.15 According to the Aldrete recovery score, the total maximum score is 10, and a score ≥9 is required for discharge. Each aspect, including the activity level, respiration, circulation, consciousness and oxygen saturation (via SpO2), was scored between 0 and 2.
The postoperative nausea and vomiting scores (0, no nausea and vomiting; 1, mild nausea, no treatment requested; 2, nausea only, give anti-emetics as prescribed until resolved; 3, vomiting, give anti-emetics as prescribed until resolved; 4, nausea/vomiting that does not respond to emetics) and side effects (such as dizziness, itching, agitation and respiratory depression) were recorded by an independent blinded observer who was unaware of the selected TCI remifentanil rate. During post-anesthesia care, the patients suffering from postoperative nausea and vomiting were treated with intravenous ondansetron (8 mg). The patients were kept in the recovery room for at least 30 minutes, and any patients who had Aldrete recovery scores of 10 and were free from postoperative nausea and vomiting were discharged.
Oral paracetamol (500 mg) was administered upon a patient's request for postoperative analgesia. The clinical and ongoing pregnancy rates were obtained from the records. Prior to their discharge, all of the women were interviewed in the recovery room about their satisfaction with the anesthetic technique and their pain after the procedure .
Statistical analysisA power analysis suggested that a minimum of 20 subjects per group would be required to provide a power of 80% for detecting a 20% change in the plasma remifentanil concentration and in the amount of remifentanil during spontaneous respiration in patients undergoing oocyte retrieval. This analysis was based on a previous study.16 The data were expressed as mean ± standard error of the mean (mean ± sem) or n (%), where appropriate. One-way analysis of variance followed by a Bonferroni correction was performed when comparing all of the continuous variables. The categorical variables were analyzed using the ×2 or Fisher's exact test, as appropriate. A p-value of less than 0.05 was considered to be statistically significant.
RESULTSThe three groups were similar with respect to their demographic data (Table 1).
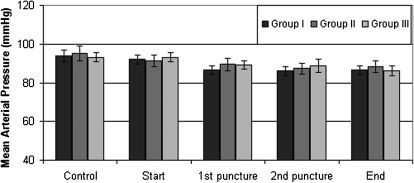
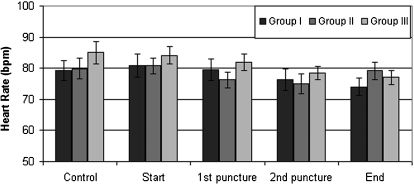
The hemodynamic variables (including the mean arterial pressure and heart rate) were comparable among the groups (Figures 1 and 2).
The duration of both the anesthesia and the procedure and the amount of propofol used with TCI were comparable among the groups, but the amount of remifentanil used in group III was significantly higher than in either group I or II, as expected from the preselected study protocol (Table 1).
The need for an increase in the remifentanil rate was observed in only 6 patients from group I, whereas none of the patients from group II or III needed increase in rate adjustments. However, we needed to decrease the remifentanil rate in 2 patients from group II and in 5 patients from group III (Table 2). These patients needed a jaw thrust followed by brief periods of assisted mask ventilation. The peripheral oxygen saturation measurements were also comparable among the groups.
Data of anesthesia protocol and pregnancy rate.
| Group I (n = 23) | Group II (n = 23) | Group III (n = 23) | |
|---|---|---|---|
| Duration of anesthesia (min) | 20.0 ± 1.8 | 17.3 ± 1.8 | 18.7 ± 1.6 |
| Duration of the procedure (min) | 11.7 ± 1.0 | 12.0 ± 1.4 | 14.6 ± 1.3 |
| Total amount of propofol (mg) | 121.9 ± 9.2 | 115.0 ± 10.3 | 134.1 ± 11.6 |
| Total amount of remifentanil (μg) | 108.7 ± 9.0 | 123.4 ± 10.2 | 164.1 ± 11.4* |
| Need for an increase in the TCI remifentanil rate [n (%)] | 6 (26) | - | - |
| Need for an decrease in the TCI remifentanil rate [n (%)] | - | 2 (9) | 5 (22) |
| Patients with an Aldrete recovery score of 10 after 5 min [n (%)] | 18 (78) | 17 (73) | 8 (35)* |
| Patients with an Aldrete recovery score of 10 after 10 min [n (%)] | 23 (100) | 23 (100) | 23 (100) |
| Postoperative nausea and vomiting (n [%] | - | 1 (4) | 2 (9) |
| Pregnancy rate (n [%] | 10 (43) | 10 (43) | 12 (52) |
Data are expressed as mean ± sem or n (%).
Five minutes after the procedure, the maximum Aldrete recovery score (10) was achieved in 18 and 17 out of 23 patients from groups I and II, respectively. However, only 8 out of 23 patients in group III reached the maximum Aldrete recovery score within 5 minutes. This number was significantly lower compared to groups I and II. However, 10 minutes after the procedure, all of the patients in the three groups had the maximum Aldrete recovery score of 10 (Table 2).
During the first 30 minutes of follow-up, none of the patients suffered from pain in the recovery room. In terms of the side effects, 1 patient from group II and 2 patients from group III suffered from postoperative nausea and vomiting (Table 2).
The clinical pregnancy rates were 43%, 43% and 52% in groups I, II and III, respectively (Table 2).
The pain assessment and sedation scores did not differ significantly among the groups (Table 3).
Sedation scores and numerical rating scales.
| Sedation score | First puncture | 5 min | 10 min | Second puncture | 15 min | 20 min | End of the procedure |
|---|---|---|---|---|---|---|---|
| Group I | 2.3±0.17 | 2.95±0.17 | 3.13±0.22 | 2.9±0.18 | 3.29±0.18 | 3±0 | 2.83±0.18 |
| Group II | 2.52±0.16 | 3.14±0.16 | 3.14±0.23 | 3.17±0.2 | 3.16±0.23 | 3±0 | 3.17±0.15 |
| Group III | 2.52±0.12 | 3.22±0.14 | 3.67±0.11 | 3.55±0.1 | 3.44±0.17 | 3.6±0.24 | 3.57±0.11 |
| Numerical rating scale | First puncture | 5 min | 10 min | Second puncture | 15 min | 20 min | End of the procedure |
|---|---|---|---|---|---|---|---|
| Group I | 0.22±0.13 | 0.7±0.3 | 1±1 | 0.24±0.17 | 0.57±0.57 | 2±0 | 0.13±0.1 |
| Group II | 0.44±0.44 | 0.29±0.17 | 0.3±0.36 | 0.53±0.21 | 0±0 | 0±0 | 0.09±0.09 |
| Group III | 0±0 | 0.35±0.19 | 0.28±0.28 | 0.23±0.13 | 0.11±0.11 | 0±0 | 0±0 |
Data are expressed as mean ±sem.
Sixty-six women who were free from postoperative nausea and vomiting stated during the interview prior to discharge that they were satisfied with their anesthetic care.
DISCUSSIONWe demonstrated that the effects of the three different protocols on hemodynamic parameters, sedation score, pain score, recovery score recorded 10 minutes after the procedure and side effects were similar. However, the percentage of women with a recovery score of 10 recorded 5 minutes after the procedure was significantly lower in the group receiving 2.5 ng/mL of TCI remifentanil with propofol due to the significantly higher amount of remifentanil in the preselected study protocol. Therefore, rates of 1.5 and 2 ng/mL of remifentanil during 1.5 μg/mL of propofol seem to be superior to the remifentanil rate of 2.5 ng/mL at providing earlier recovery.
When conscious sedation with remifentanil infusion was compared with conscious sedation with a paracervical block, an extra benefit was not found, although the plasma remifentanil concentrations calculated by the software showed a significant decrease in the group receiving a paracervical block and remifentanil.16 Additionally, it has been reported that the use of 50% oxygen/nitrous oxide via mask ventilation with TCI propofol reduces the amount of propofol that is required to prevent a response to oocyte retrieval in 50% of women (compared to women receiving oxygen/air).13
The target-controlled infusion system developed in the last decade rapidly provides stable effect-site concentrations that depend on the drug onset time and are maintained for as long as they are desired because these devices deliver intravenous drugs using a computer-controlled algorithm that considers each drug's particular pharmacokinetic and pharmacodynamic properties.14,17,18 TCI allows the user to rapidly achieve a chosen predicted concentration without overshooting, and both drugs can be precisely titrated in the narrow therapeutic window between agitation and excessive sedation.6,19
Remifentanil has properties that are desirable for a sole or adjunct conscious sedation agent, such as rapid onset and recovery, whereas propofol provides rapid onset, rapid recovery, and anxiolysis.7,11,13,20,21 Although propofol and remifentanil are suitable for providing controlled sedation and analgesia, deep sedation can result in airway loss with serious consequences, particularly in spontaneously breathing patients.8 To control sedation and analgesia, we adjusted the remifentanil rates in the present study. Because the numerical rating scale was found to be higher than 3 during TCI remifentanil at Ce 1.5 ng/mL in 26% of the patients in group I, we increased the remifentanil rate by 0.5 ng/mL in these patients. However, TCI remifentanil at Ce 2 or 2.5 ng/mL was adequate to maintain satisfactory sedation/analgesia. Additionally, we decreased the remifentanil rate in 9% and 22% of patients receiving 2 and 2.5 ng/mL, respectively. In these patients, airway support was provided with a jaw thrust followed by brief periods of assisted mask ventilation. We did not find any significant difference in the pain scores or peripheral oxygen saturation results among the groups, although rate adjustments were performed in the patients with a numerical rating scale greater than 3 and an SpO2 that was <95%. This could be explained by the rate (0.5 ng/mL) that we chose to use to increase or decrease the adjustments, which could have caused such patients to be a part of another group that had already existed in the study.
The initial TCI remifentanil and propofol rates may vary depending on the type of anesthesia, such as conscious sedation vs. general anesthesia. Initial remifentanil rates of Ce 1, 1.5 and 3 ng/mL (using the Minto pharmacokinetic model) have been utilized in combination with initial propofol rates of Cp 0.8, 1, 2, 2.5 and 4 μg/mL in several outpatient procedures.4, In studies on oocyte retrieval, TCI propofol without remifentanil was used at Cp 2.5 μg/mL.11,12 However, none of these studies have investigated the efficacy of TCI remifentanil and propofol during oocyte retrieval. Currently, we compared effect site TCI remifentanil at 1.5, 2 and 2.5 ng/mL with 1.5 μg/mL of propofol to find the optimal regimen for these two drugs in combination. Consequently, our study is the first study to demonstrate that TCI remifentanil at Ce 1.5, 2 or 2.5 ng/mL with 1.5 μg/mL of propofol provides satisfactory conscious sedation and analgesia without hemodynamic alterations during oocyte retrieval, assuming that rate adjustment for TCI remifentanil is not considered an issue.
The potential limitations of our study are not selecting objective criteria for TCI rate adjustment and not using bispectral index monitoring. Further studies might be designed to vary both TCI propofol and remifentanil to benefit from the merits of administering these pharmacologically suitable drugs, even in short procedures.
The present TCI propofol rate (Ce 1.5 μg/ml) was less than that in both studies by Hong et al.,11,12 in which propofol was used without remifentanil, and the initial propofol rate was 2.5 μg/ml for oocyte retrieval. The reason why we selected a lower propofol rate than that used in these studies was that propofol was used in combination with remifentanil instead of as a sole agent. Moreover, we had the opportunity to select the effect-site mode of the latest TCI device (Base Primea), which offers a faster onset and easier titration than the old device (Diprifusor), which was used to administer an initial propofol rate of 0.8 μg/mL with an initial remifentanil rate of Ce 1 ng/ml during pregnancy termination in an observational non-randomized prospective study.10 Using the current three regimens, 100% of the patients completely recovered in the recovery room 10 minutes after the procedure in this prospective randomized study.
Nitrous oxide has been used with propofol (via Diprifusor) at a Cp of 4 μg/mL during transvaginal ultrasound-guided oocyte retrieval. It has been reported that the Cp 50 values were significantly lower in patients receiving a 50% nitrous oxide/oxygen mixture than in those receiving a 50% oxygen/air mixture, and the pregnancy rates were comparable between the groups.13 No detrimental effects on reproductive outcomes have been found with remifentanil and propofol.22,23 Although the clinical pregnancy rate was not a primary outcome of our study, we observed comparable clinical pregnancy rates among the groups. However, the rates we observed were almost two-fold higher than the reported rates.
The potential for postoperative nausea and vomiting associated with remifentanil is dose-related.15 We observed that 1 and 2 patients in groups II and III, respectively, suffered from these side effects. The higher remifentanil rate in group III did not result in a statistically significant increase in the incidence of postoperative nausea and vomiting. Other potential side effects of remifentanil, including dizziness, itching and pruritus, were not observed in our study.
In conclusion, TCI remifentanil at Ce 1.5 and 2 ng/mL with propofol at Ce 1.5 μg/mL may be superior at providing an earlier recovery than remifentanil at Ce 2.5 ng/mL, although satisfactory and relatively pain-free conscious sedation without hemodynamic parameter alterations were achieved with all three regimens for oocyte retrieval.