Hypoplastic left heart syndrome (HLHS) inevitably results in death within a few weeks of birth, and the surgical treatment for this condition involves three complex surgeries that are associated with a high mortality and cost (1). The mortality rates for the first-stage procedure range from 25-80% at medical centers worldwide. Moreover, many children (possibly as many as 50%, according to hospitals where accurate records are kept) with this disease are not referred for surgery and progress to death within days or weeks (2–4). The two conventional techniques that are used for the Norwood procedure (the first-stage surgical correction) involve the construction of a systemic pulmonary shunt. This procedure performed using the Blalock-Taussig technique leads to a diastolic systemic pulmonary flow that mimics systemic valve insufficiency. When performed using the Sano modification technique, the Norwood procedure involves building a tube between the systemic ventricle and the pulmonary artery; this technique avoids the diastolic systemic pulmonary flow but allows the blood in the lungs to reflux to the single ventricle, which increases the volume overload. This overload associated with ventriculotomy may lead to dysfunction of the ventricle and arrhythmia. Moreover, both techniques necessitate the use of an artificial tube and carry a risk for thrombosis or stenosis (5–9). Additionally, fabrication of the neo-aorta is a laborious technique that requires the extended use of extracorporeal circulation (ECC).
Hybrid approaches involving the maintenance of the patent ductus arteriosus with the implantation of a stent (10,11) or the prolonged administration of prostaglandin E1 (12) associated with the banding of the pulmonary branches simplifies the surgical approach but have yielded unsatisfactory results (13,14).
PROPOSED PROCEDURE FOR THE CORRECTION OF HLHS, STAGE 1- 1.
Typical preparations for an intracardiac operation with ECC.
- 2.
Median sternotomy.
- 3.
Dissection of the brachiocephalic trunk and implantation of a 3.5-mm Gore-Tex graft for arterial ECC access.
- 4.
Dissection and cannulation of the femoral artery as a second route of arterial access to maintain systemic perfusion after aortic clamping.
- 5.
Cannulation of both venae cavae.
- 6.
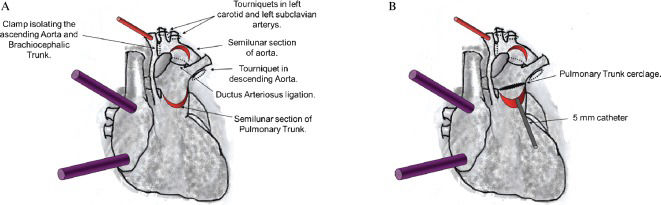
Placement of tourniquets in the left carotid artery, left subclavian artery and descending aorta after the insertion of the ductus arteriosus.
- 7.
Initiation of ECC and ligation of the ductus arteriosus.
- 8.
Placement of a clamp at the aortic arch to isolate the ascending aorta and the brachiocephalic trunk from the systemic circulation, which serves to maintain the cerebral, right superior limb and coronary perfusion.
- 9.
Closure of the left carotid artery, left subclavian artery and descending aorta tourniquets with the continued maintenance of the descending aortic circulation via the femoral cannula.
- 10.
Autologous tissue is formed into a flap using a semilunar section of the initial portion of the anterior pulmonary trunk 3 cm above the pulmonary valve (Figure 1).
Figure 1.A) Cannulation of the brachiocephalic trunk (cannulation of femoral artery not showed in the figure) and double venae cavae for extracorporeal circulation (ECC). Placement of the tourniquet in the left carotid and left subclavian arteries and descending aorta. Subsequently, ECC is initiated, and the ductus arteriosus is ligated. A clamp is then placed at the aortic arch to isolate the ascending aorta and the brachiocephalic trunk from the systemic circulation. Next, the tourniquets are closed, and a semilunar section of the initial portion of the anterior pulmonary trunk (3 cm above the pulmonary valve) is used to create a flap. A second semilunar incision is made into the anterosuperior side of the aortic arch near the left carotid and left subclavian arteries to create a second flap. B) A 5-mm catheter is introduced through the opening of the pulmonary trunk towards the pulmonary branches. The pulmonary trunk cerclage is obtained by suturing a cardiac tape around the catheter with 5-0 prolene.
(0.03MB). - 11.
A second semilunar incision is made into the anterosuperior side of the aortic arch near the left carotid and left subclavian artery to create a second flap of autologous tissue (Figure 1A).
- 12.
Introduction of a 5-mm catheter through the opening of the pulmonary artery towards the pulmonary branches. Around this catheter is placed the pulmonary trunk cerclage using cardiac tape sutured with 5-0 prolene. This procedure results in a systemic pulmonary shunt with autologous tissue (Figure 1B).
- 13.
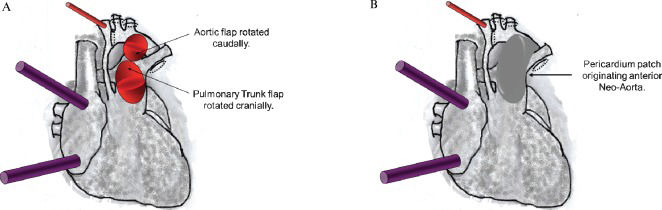
The free edges of the semilunar flaps of the aorta and pulmonary trunk are rotated and sutured edge-to-edge, originating the autologous posterior neo-aortic wall (Figure 2A). A second line of stitches is placed to dorsally exclude the ductus arteriosus tissue.
Figure 2.A) The free edges of the semilunar flaps of the aorta and pulmonary trunk are rotated and sutured edge-to-edge originating the autologous posterior neo-aortic wall. B) A valved bovine pericardium patch is implanted from the anterior opening of the pulmonary trunk to the aortic arch and extends to the beginning of the descending aorta to give rise to the anterior wall of the neo-aorta.
(0.02MB). - 14.
A valved bovine pericardium patch is implanted from the anterior opening of the pulmonary trunk to the aortic arch and extends to the beginning of the descending aorta to give rise to the anterior wall of the neo-aorta (Figure 2B). The opening of the valve is placed near the lower edge of the aortic arch. This valve prevents diastolic systemic reflow to the pulmonary arteries and improves coronary perfusion pressure through the ascending aorta (retrograde flow). The neo-aorta does not require mobilization of the ascending aorta or the pulmonary trunk.
- 15.
A small right atriotomy is performed for atrial septum resection. After the appropriate maneuvers are performed to remove air that may be present, the tourniquets are released for subsequent finalization of the ECC.
- 16.
The remainder of the procedure is performed according to the conventional protocol.
The procedure was simulated in an experimental mechanical model as described below.
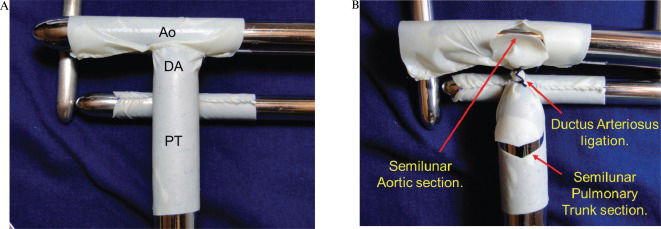
The model was constructed with bovine pericardium tissue (HP Bioprosthesis Ltda.). The pulmonary trunk was represented using an 18-mm pericardial tunnel. On its posterior superior face, a second 12-mm pericardial tunnel was placed to simulate the pulmonary branches. On the cranial face of the pulmonary trunk, an additional 18-mm pericardium tube was placed to simulate the aortic arch. The pulmonary trunk segment between the pulmonary branches and the aorta represented the ductus arteriosus. All anastomoses were performed with prolene 6-0 (Figure 3A).
A) The pulmonary trunk (PT) was represented using an 18-mm pericardial tunnel. A second 12-mm pericardial tunnel is placed on its posterior superior face to simulate the pulmonary branches. An additional 18-mm pericardium tube, which simulated the aortic arch (Ao), is then placed on the cranial face of the pulmonary trunk. The pulmonary trunk segment between the pulmonary branches and the aorta represented the ductus arteriosus (DA). B) The DA is ligated, and the autologous flaps were obtained through the semilunar opening of the aorta and pulmonary trunk.
The ductus arteriosus was ligated. The semilunar openings of the aorta and pulmonary trunk used to form the autologous flaps (to construct the neo-aorta) were created as depicted in Figure 3B).
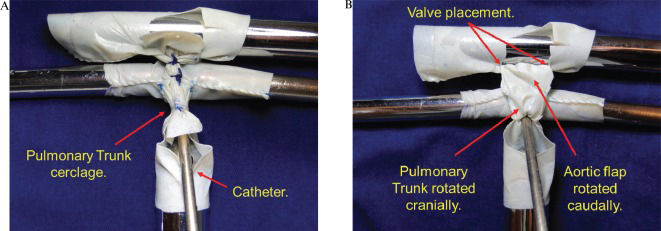
A 5-mm catheter was introduced through the opening of the pulmonary trunk towards the pulmonary branches. The pulmonary trunk cerclage was obtained by suturing a cardiac tape around the catheter with 5-0 prolene (Figure 4A). Following the removal of the catheter, a 5-mm systemic pulmonary shunt of autologous tissue was created.
A) Through the opening of the pulmonary trunk, a 5-mm catheter is introduced towards the pulmonary branches. The pulmonary trunk cerclage is obtained by suturing a cardiac tape with 5-0 prolene around the catheter. B) The aortic flap is rotated caudally, and the pulmonary trunk flap is rotated cranially. These flaps are then sutured edge-to-edge with 6-0 prolene originating the autologous posterior neo-aortic wall. The red marks indicate where the top of the valve of the pericardium should be placed.
The aortic flap was rotated caudally, and the pulmonary trunk flap was rotated cranially. These flaps were then sutured edge-to-edge with 6-0 prolene originating the autologous posterior wall of the neo-aorta. There was no tension in this suture, and no mobilization of the aortic arch was required (Figure 4B).
The anterior wall of the neo-aorta was created using a bovine pericardium patch sutured with prolene 6-0 (Figure 5). The valved patch was not used in this model, although the location of the valve position is shown in Figure 4B). The black dashed line indicates where the valve suture would be inserted into the pericardial patch that forms the anterior wall of the neo-aorta.
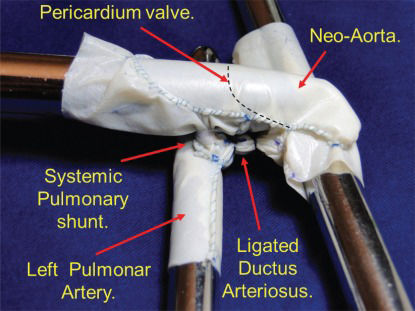
The implantation of a bovine pericardium patch from the anterior opening of the pulmonary trunk to the aortic arch. This patch extends to the beginning of the descending aorta and gives rise to the anterior wall of the neo-aorta. The dashed line represents the valve suture on the pericardium.
The construction of an experimental model used to demonstrate the novel technique for the correction of HLHS proved to be simple.
The proposed procedure requires fewer sutures, which excludes the need to implant a heterologous systemic pulmonary shunt and decreases the surgical time. One patient who met the relevant criteria and required surgical correction was submitted to the procedure (15) and demonstrated that it could potentially reduce the perioperative complications.
The technique described does not require any period of coronary ischemia or surgical manipulation of the ventricle, as described previously (15). The combined effect of these improvements should promote a higher quality of postoperative ventricular function. Moreover, maintenance of the anatomy of the ascending aorta simplifies the procedure and avoids distortions in the coronary arteries.
The in situ autologous systemic pulmonary shunt is simple to manufacture and should not generate thrombosis-related complications. In addition, the maintenance of the pulmonary trunk in situ (with the cerclage) should promote better development of the pulmonary tree.
The use of a valved pericardium for the creation of a neo-aorta should improve systemic perfusion (including the coronary circulation) and prevent diastolic systemic pulmonary reflow.
No potential conflict of interest was reported.