A low ratio of omega-6/omega-3 polyunsaturated fatty acids is associated with healthy bone properties. However, fatty diets can induce obesity. Our objective was to evaluate intra-abdominal adiposity, insulin, and bone growth in rats fed a high-fat diet containing low ratios of omega-6/omega-3 provided in canola oil.
METHODS:After weaning, rats were grouped and fed either a control diet (7S), a high-fat diet containing soybean oil (19S) or a high-fat diet of canola oil (19C) until they were 60 days old. Differences were considered to be significant if p<0.05.
RESULTS:After 60 days, the 19S and 19C groups showed more energy intake, body density growth and intra-abdominal fat mass. However, the 19S group had a higher area (200%) and a lower number (44%) of adipocytes, while the 7S and 19C groups did not differ. The serum concentrations of glucose and insulin and the insulin resistance index were significantly increased in the 19C group (15%, 56%, and 78%, respectively) compared to the 7S group. Bone measurements of the 19S and 19C groups showed a higher femur mass (25%) and a higher lumbar vertebrae mass (11%) and length (5%). Computed tomography analysis revealed more radiodensity in the proximal femoral epiphysis and lumbar vertebrae of 19C group compared to the 7S and 19S groups.
CONCLUSIONS:Our results suggest that the amount and source of fat used in the diet after weaning increase body growth and fat depots and affect insulin resistance and, consequently, bone health.
Obesity, a worldwide public health problem, usually begins early in life, persists into adulthood and significantly increases the risk for morbidity from dyslipidemia, type-2 diabetes mellitus, and coronary heart disease.1–3 These endocrine-metabolic disturbances can also interfere with bone remodeling.4 Although genetic composition is a major contributor to peak bone mass, lifestyle factors (such as diet) also contribute to the attainment of peak bone mass.5,6
Despite the metabolic repercussions of obesity, extensive epidemiological data have shown that a high body weight or body mass index (BMI) is associated with increases in bone mass and a reduced risk for fractures.7–9 This event occurs directly via mechanical loading and indirectly via hormonal production by adipocytes or insulin.10,11 In contrast, investigators have suggested that fat mass may or may not be associated with bone mass.12,13 Given these discrepancies, the effect of fat tissue on bone health is far from clear.
Adipocytes and osteoblasts share the same mesenchymal precursor; studying adipocyte/osteoblast balance represents a challenge to treating adipose tissue and bone disorders.14 In recent years, a growing body of evidence has supported the notion that dietary long-chain polyunsaturated fatty acids (PUFAs) with a chain length longer than 18C are beneficial for bone health.15 In addition, a higher ratio of omega-6 (linoleic acid, 18:2n-6) to omega-3 (alpha linolenic acid, 18:3n-3) fatty acids is associated with detrimental bone health effects, and a lower ratio is associated with healthy bone properties.16 These PUFAs can induce obesity by acting directly on preadipocytes, mainly increasing the rate of replication and/or differentiation17 in the early stages of adipose tissue development.18 Although the role of a high-fat diet in obesity development have been extensively studied, the role of dietary fats on bone development has only recently emerged as an interesting area of research.5,6,17–20
Previously, we reported the effects of a normocaloric diet containing canola oil on adiposity and bone growth in young rats.21 Canola oil (7%) seems to beneficially decrease both abdominal adiposity and insulin resistance. However, it reduces bone density compared to 7% soybean oil. However, to the best of our knowledge, there have been no studies with an experimental model of bone metabolism in young rats fed a high-fat diet containing canola oil. In Brazil, the population consumption of soybean oil, which is rich in polyunsaturated fatty acids (ratio omega-6/omega-3 = 6.75), represents 82% of the calories originating from fat sources, while canola oil (ratio omega-6/omega-3 = 1.90) represents less than 4%.22,23
The aim of this study was to evaluate the body growth and bone health of young adult animals fed a high-fat diet containing soybean or canola oil after weaning.
MATERIALS AND METHODSThe protocol to use and handle the experimental animals was approved by the Ethics Committee of the Biology Institute of the State University of Rio de Janeiro, based on the principles adopted and promulgated by Brazilian law concerning the rearing and use of animals in teaching and research activities in Brazil.24
Wistar rats were kept in a room under a controlled temperature (25±1°C) and an artificial dark-light cycle (lights on from 07:00 to 19:00 hours). Virgin female rats (three months old) were caged with male rats, and, after mating, each female was placed in an individual cage with free access to water and food until delivery.
Within 24 h of birth (day 0), any excess pups were removed, such that only six male pups were kept per dam. This procedure has been shown to maximize lactation performance.25 During the 21 days of lactation, the rat dams were continued on an ad libitum diet of standard laboratory food (Agroceres®, São Paulo).
Male Wistar rats from six different litters were randomized and grouped on postnatal day 21 to receive either a control diet containing 7 ml of soybean oil and 54 g of cornstarch/100 g (7S group; n = 12) or a high-fat diet containing either 19 ml of soybean (19S group; n = 12) or canola oil (19C group; n = 12) along with 42 g of cornstarch/100 g. The 7S and high-fat diet groups received the same amounts of vitamins and minerals per gram of diet (Table 1). The diets were manufactured once a week and stored as pellets at 4°C in agreement with American Institute of Nutrition (AIN-93G) recommendations.26,27 The energy intake (kcal/day) and body density (body mass [g] divided by the length [cm, measured as the distance from tip of the nose to the tip of the tail])28 were evaluated in all pups every three days. All groups had free access to diet and water during the course of experimental period.
The compositions of the experimental diets.
| Ingredient (g/100 g) | 7S | 19S | 19C |
|---|---|---|---|
| Casein | 20 | 20 | 20 |
| Corn starch | 52.95 | 40.63 | 40.63 |
| Sucrose | 10 | 10 | 10 |
| Soybean oil | 7 | 19.32 | |
| Canola oil | --- | --- | 19.32 |
| Fiber | 5 | 5 | 5 |
| AIN-93G mineral mix | 3·5 | 3·5 | 3.5 |
| AIN-93 vitamin mix | 1 | 1 | 1 |
| L-Cysteine | 0·3 | 0·3 | 0.3 |
| Choline bitartrate | 0.25 | 0.25 | 0.25 |
| Energy | |||
| Kcal/g | 4.7 | 5.8 | 5.8 |
| Protein (% of energy) | 17 | 14 | 14 |
| Carbohydrate (% of energy) | 65 | 45 | 45 |
| Fat (% of energy) | 17 | 39 | 39 |
7S, the control group fed a diet containing 7 ml/100 g soybean oil; 19S and 19C, the experimental groups fed diets containing 19 ml/100 g soybean or canola oil, respectively.
casein; mineral and vitamin mix; L-cysteine; choline bitartrate: Agroquímica®; corn starch: Cargill®; fiber: Natural Pharma®; soybean and canola oil: Proquímios®; commercial sucrose: União®.
Formulated to meet the American Institute of Nutrition AIN-93G recommendations for rodent diets.25
At 59 days of age, the rats were deprived of food overnight, and, the next morning (at 60 days), the fasting rats were anesthetized with a lethal dose of pentobarbital. Blood was obtained by cardiac puncture. The abdominal fat mass, right femur and lumbar spine were excised. The blood samples were centrifuged to obtain the serum, which was stored at -20°C for a posterior analysis of glucose, calcium, and phosphorus by the colorimetric method (Bioclin, Belo Horizonte, MG, Brazil). The serum insulin concentration was analyzed using an RIA kit in only one assay (Linco Research, Inc., St. Charles, MO, USA). To determine the insulin sensitivity of the animals, we used the insulin resistance index (IRI), defined as the fasting insulin (μUI/ml) × the fasting glucose (mmol/l).
The abdominal fat depots were dissected and weighed, and the values were expressed in grams (g). The samples of retroperitoneal fat were collected and fixed in buffered formaldehyde. The tissues were embedded in paraffin, cut into 5 μm sections and stained with hematoxylin-eosin. For the morphometric analyses, profiles with at least 100 adipocytes were randomly selected and captured for each animal. The cross-sectional area (μm2) and number (per 100 μm2) of adipocytes were determined from the digital images acquired (TIFF format, 36 bit color, 1360×1024 pixels) with an Optronics CCD video camera system and an Olympus BX40 microscope and analyzed with Image-Pro Plus version 5.0 software (Media Cybernetics, Silver Spring, MD, USA).21 Two different observers independently evaluated the images and obtained similar results.
The right femur and lumbar vertebrae (LV1-LV6) were cleaned to remove any soft tissue. The distance between the epiphysis29 and the LV1-LV65 and the medial-point diaphysis width (mm for both measurements) were measured using calipers (0.01-mm readability) and stored in saline solution at -20°C until analysis. After a single scan by computed tomography (CT, Helicoidally model HISPEED, GE®), the images were obtained from 5-mm-thick axial slices. The radiodensity (expressed as Hounsfield units, HU) of the proximal epiphysis and the diaphysis of the femur and the mean cross-sectional areas of the lumbar vertebrae were measured using computerized analysis software (DicomWorks v1·3·5, 2002) by manual selection of the region of interest.30 After the computed tomography analyses, the femur and LV1-LV6 were dried overnight at 95°C and weighed (mg).31
Statistical analyses were performed using the GraphPad Prism statistical package (version 5.00, 2007, San Diego, CA, USA). The energy intake and body density were analyzed using two-way ANOVA followed by post hoc Bonferroni tests. The remaining results were analyzed using one-way ANOVA followed by post hoc Newman-Keuls tests. All of the results are expressed as means±SEM with a significance level of 0.05.
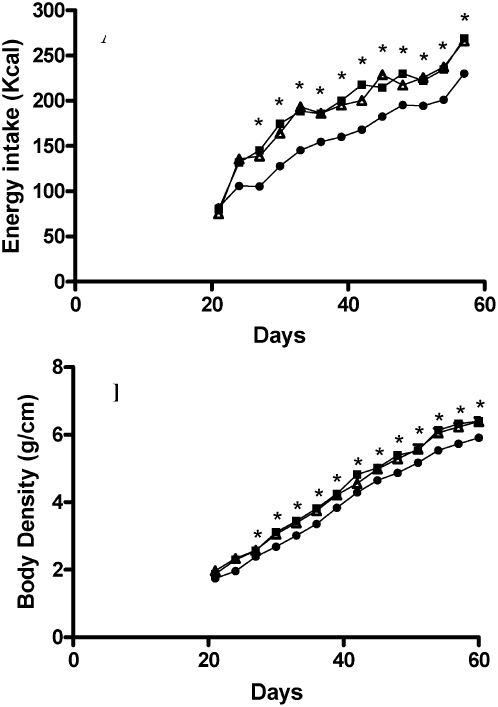
RESULTSAfter weaning, the 19S and 19C groups showed similar energy intake and body density growth, but these were significantly increased compared to the control group at 27 days of age (Figure 1).
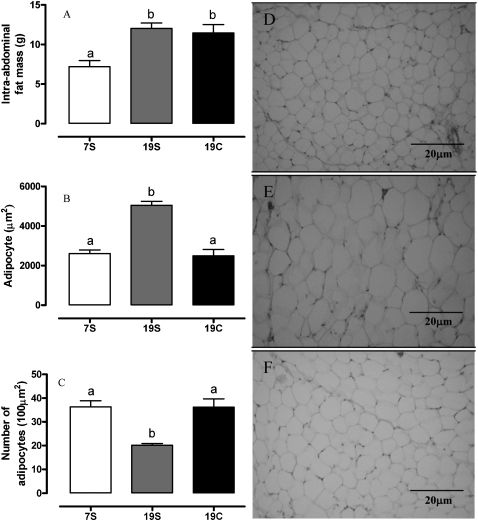
The intra-abdominal fat mass did not differ between the 19S (12.0±0.6 g) and 19C (11.4±1.0 g) groups; however, it was significantly higher (p<0.05) compared to the 7S group (7.2±0.7 g). The morphometric analyses of the adipocytes revealed larger cells in the 19S group (5048±201.2 μm2; p<0.0001) than in the 7S (2607±186.3 μm2) and 19C (2489±322·7 μm2) groups. However, the number of adipocytes in the 19S (20.17±0.73) group was significantly less than in the 7S (36.33±2.59) and 19C (36.18±3.52) groups (Figure 2).
(A) Intra-abdominal fat mass. (B) Adipocyte size and (C) number of retroperitoneal adipocytes. Groups fed with control diet (7S, n = 12) or with high fat diet containing soybean (19S, n = 12) or canola oil (19C, n = 12), at 60 days. a,bValues with different superscripts are significantly different (one-way ANOVA; p<0.05). Photomicrographs of the adipose tissue staining with Hematoxylin-Eosin (original magnification 200X): (D) 7S, (E) 19S and (F) 19C groups.
The serum analyses did not reveal any differences in the calcium and phosphorus concentrations. The 19C group showed significantly higher concentrations of glucose (+15%) and insulin (+56%) and an increased insulin resistance index (+78%) compared to the 7S group. The 19S group did not differ from 7S and 19C groups (Table 2).
Serum analyses after 60 days.
| 7S | 19S | 19C | |
|---|---|---|---|
| Calcium, mg/dL | 9.8±0.2 | 9.3±0.1 | 9.4±0.2 |
| Phosphorus, mg/dL | 9.5±0.4 | 10.4±0.4 | 8.8±0.3 |
| Glucose, mg/dL | 97.3±3.5a | 104.7±2.8a,b | 112.0±3.6b |
| Insulin, μUI/ml | 37.6±4.3a | 43.5±2.5a,b | 58.4±7.3b |
| Insulin Resistance Index (IRI) | 203.3±0.8a | 252.0±0.4a,b | 363.0±1.4b |
The post-weaning groups were fed a control diet (7S; n = 12) or a high-fat diet containing either soybean (19S; n = 12) or canola oil (19C; n = 12) until they were 60 days old.
Differences in the femur mass between the 19S and 19C groups were not found. However, they were higher (+25%; p<0.05) in these groups than in the C group. The femur measurements showed that the distance between the epiphyses was similar in all of the groups, and the width of the diaphysis was higher in the 19C group (+9%; p<0.05). The mass and the length of lumbar vertebrae did not differ between the 19S and 19C groups, but they were higher (+11% and +5%; p<0.05, respectively) in these groups than in the 7S group (Table 3).
Femur and lumbar vertebrae (LV1-LV6) measurements after 60 days.
| 7S | 19S | 19C | |
|---|---|---|---|
| Femur: | |||
| Mass, mg | 355.1±22a | 444.8±10.3b | 444.2±9.4b |
| Distance between epiphysis, mm | 31.5±0.6 | 31.9±0.3 | 31.9±0.3 |
| Width of the diaphysis, mm | 3.2±0.1a | 3.25 ±0.1a,b | 3.51±0.1b |
| Lumbar vertebrae: | |||
| Mass, mg | 613.8±24.8a | 689.7±11b | 681.9±17.4b |
| Maximum length, mm | 37.7±0.4a | 39.9±0.3b | 39.5±0.4b |
The post-weaning groups were fed a control diet (7S; n = 12) or a high-fat diet containing soybean (19S; n = 12) or canola oil (19C; n = 12) until they were 60 days old.
Evaluating the femur using computed tomography (CT) showed that the radiodensity of the proximal epiphysis in the 19C group was significantly higher than in the 19S and control groups (+20% and +27%, respectively). The radiodensity of the diaphysis did not differ between the 19S and 19C groups, but these groups had significantly higher (+15%) diaphyseal radiodensities compared to the control group. The lumbar vertebrae analysis of the 19S and 19C groups using CT showed an increased radiodensity (+17% and +29%; p<0.05, respectively). Simultaneously, the 19C group was greater lumbar radiodensity (+9%; p<0.05) than the 19S group when comparing the high-fat groups (Table 4).
Computed tomography analyses of the femur and lumbar vertebrae (LV1-LV6) after 60 days.
| 7S | 19S | 19C | |
|---|---|---|---|
| Proximal epiphysis, Hu | 546.0±37.5a | 515.7±34.7a | 658.1±24.5b |
| Diaphysis, Hu | 492.6±21.2a | 562.2±24.4b | 579.4±22.1b |
| LV1-LV6, Hu | 389.9±14.2a | 459.0±7.1b | 502.4±4c |
The post-weaning groups were fed a control diet (7S; n = 12) or a high-fat diet containing soybean (19S; n = 12) or canola oil (19C, n = 12) until they were 60 days old.
Dietary fat is calorie-dense and extremely palatable. It is easily overconsumed because it can cause less satiety than carbohydrates and protein,17 causing “high-fat hyperphagia.”32 However, we did not verify hyperphagia when the rats were fed a high-fat diet containing soybean or canola oil after weaning.33 Nevertheless, the increase in the diet lipids' caloric percentile from 17% to 39% contributed to increased body density growth and larger intra-abdominal fat depots in the 19S and 19C groups, independent of the vegetable oil type and absence of hyperphagia.
Hyperlipidic diets affect cell morphology, hormone sensitivity, and gene expression within the preexisting adipocytes in a complex manner. These lead to the recruitment of adipocyte precursor cells, initiating differentiation and producing the infrastructure required to sustain the new tissue.34,35 In our experimental model, despite the similar gains in body density and intra-abdominal fat mass, the 19S group showed an increase in the size and a decrease in the number of retroperitoneal adipocytes compared to the 19C group. Other authors studying models of obesity induced by a high-fat diet intake have observed that the development of body fat compartments and the adipocyte fatty acid composition are affected by the type of fat contained in the diet.36–39 When a high-fat diet containing omega-6 is consumed, peroxisome proliferator-activated receptor gamma (PPARγ) helps to convert unspecialized cells into adipocytes to store extra fat.40 Interestingly, high-fat diets containing omega-3 (PUFA) limit post-intake fat storage and adipocyte hypertrophy.41 In contrast, canola oil (when compared to soybean oil) is characterized by very low levels of omega-6 (21% vs. 54%, respectively) and high levels of omega-3 (11% vs. 8%, respectively).23 Thus, these pathways help to explain the cell size distribution of the retroperitoneal adipocytes.
From the bone analysis, the increases in the body density and abdominal fat depots might be associated with the increases in the femur mass, the lumbar vertebrae mass and length, and the radiodensity of diaphysis and lumbar vertebrae in the groups fed a high-fat diet. Some human and experimental studies have revealed a positive relationship between body weight or body mass index (BMI) and bone mass. This is mediated by mass mechanical stress, which is important for remodeling bone architecture10,42–44 and providing stimuli for osteogenesis.6 Our results agree with previous literature that reported positive effects of fat mass on bone density. Furthermore, adipose tissue might influence bone density by promoting bone-active hormone secretion from the pancreas (e.g., insulin).11
The serum analyses revealed high concentrations of insulin and glucose and, consequently, high insulin resistance in rats fed a high-fat diet containing canola oil. These results are surprising because omega-6 has been associated with the development of type-2 diabetes mellitus.39 However, human and animal studies have revealed that excessive fat intake might induce metabolic disturbances independent of the diet lipid composition.45–50 Thus, a high-fat diet containing 19% canola oil seems to promote insulin resistance. The fatty acid composition and the size and number of adipocytes are likely stronger contributors to insulin resistance than fat mass. Our data regarding fat cell morphology are based on retroperitoneal samples; therefore, further studies using mesenteric, epididymal and subcutaneous fat are required to elucidate the mechanisms that explain the association between canola oil intake and insulin sensitivity. Furthermore, increased bone density has been associated with hyperinsulinemia in non-diabetic models.51,52 Insulin is a potent regulator of bone growth, acting directly on osteoblasts by stimulating their proliferation and, consequently, inducing bone formation.11,53 Although this study has no data to confirm osteoblast activity, we hypothesized that the hyperinsulinemia observed in the 19C group may have had a positive effect on the bones, increasing their radiodensities.
Bone density increases more predominantly at the trabecular site (i.e., the vertebral body) than at the cortical site (i.e., the proximal epiphysis), and bone remodeling explains these regional differences.53 When we evaluated each bone individually, we found that high canola oil intake promotes similar trends in the proximal epiphysis and in the lumbar vertebrae, increasing bone radiodensity. These analyses indicate that the use of computed tomography for bone analysis enables the differentiation between the 19S and 19C groups that is impossible using other bone measurements.
The results suggest that the amount and source of fat in the diet after weaning have differential effects on adiposity and bone. When the canola oil diet is normocaloric, a lower intra-abdominal adiposity and lower bone density result. 21 Thus, regardless of the source (soybean or canola oil), a high-fat diet ameliorates bone quality and induces adiposity. However, canola oil also causes hyperinsulinemia in this model. Thus, public policies to adopt various oils in the diet of the population must consider the deleterious effects of higher fat contents.
The authors thank Mr. C. Roberto for providing animal care. This research was supported by the State of Rio de Janeiro Carlos Chagas Filho Research Foundation (FAPERJ) and the Coordination for the Enhancement of Higher Education Personnel (CAPES).
No potential conflict of interest was reported.