The Japanese ABO-Incompatible Transplantation Committee officially collected and analyzed data on pediatric ABO-incompatible living-donor kidney transplantation in July 2012. The age of a child was defined as <16 years, and 89 children who had undergone ABO-incompatible living-donor kidney transplantation from 1989 to 2011 were entered in a registry. These data were presented as the Japanese registry of pediatric ABO-incompatible living-donor kidney transplantation at the regional meetings of the International Pediatric Transplantation Association (IPTA) in Nagoya in September 2012 and in Sao Paulo in November 2012.
ABO-incompatible (ABOi) living-donor kidney transplantation (ABOiLDKTx) has been adopted for children and adults. In the Japanese ABOiLDKTx registry, 89 of 2,218 recipients of ABOiLDKTx were children aged <16 years. The donors were primarily parents (n = 86; 98%). Overall graft survival rates in children (aged <16 years) and adults (aged ≥16 years) were, respectively, 94% and 93% at 1 year, 90% and 85% at 5 years, and 85% and 70% at 10 years post-transplantation. Consequently, the graft survival rate in children was significantly better than in adults (p<0.05). Graft survival rates in children administered cyclosporine (CsA; n = 41) or tacrolimus (FK; n = 48) were 95% and 94%, respectively. No significant difference in patient or graft survival rates was observed between children administered CsA and those administered FK.
In addition, we retrospectively studied pediatric ABOiLDKTx in the Department of Nephrology and Pediatric Nephrology, Toho University Omori Medical Center, as a single-center study. In total, 21 children (<20 years of age, as defined by the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) underwent ABOiLDKTx at the Toho University Omori Medical Center. Graft and patient survival rates were both 95% 10 years post-Tx. The administration of rituximab (RIT) without splenectomy was effective for desensitization pre-Tx to suppress anti-donor blood-group antibody (ADBGAB) IgG. ABOiLDKTx should be considered for children with end-stage renal failure.
The outcome of ABOiLDKTx was not expected to be favorable >20 years ago because it was commonly believed that hyperacute rejection could occur in any recipient with a blood group incompatible with that of the donor. However, the incidence of hyperacute rejection is incredibly low (1), and this rejection never occurs if RIT, mycophenolate mofetil (MMF), a steroid, and a calcineurin inhibitor (CNI) are used as desensitizing agents before transplantation. ABOiLDKTx is now very popular in Japan because its outcomes have been demonstrated to be similar to those of ABOcLDKTx (2,3). In addition, pediatric KTx has been adopted for ABOiLDKTx. We collected and analyzed data from all pediatric kidney transplant centers to generate a registry of pediatric ABOiLDKTx under the Japanese ABO-Incompatible Transplantation Committee. We also studied pediatric ABOiLDKTx at the Toho University Omori Medical Center as a single-center study. Children with end-stage renal failure tend to become growth retarded. In addition, peritoneal dialysis cannot be continued in children for an extended period. Therefore, ABOiLDKTx should be considered earlier or preemptively for children.
PATIENTS AND METHODSRecipients and donorsIn the Japanese registry, the recipient and donor ages at the time of ABOiLDKTx were 11.1±3.0 years and 41.4±6.7 years, respectively. The recipients included 54 boys (61%) and 35 girls (39%), and the donors included 27 males and 62 females. In total, 86 parents (98%) and three other relatives acted as donors for the recipients. The control group comprised 2,129 adult recipients of ABOiLDKTx who were ≥16 years of age.
The recipient and donor ages at the time of ABOiLDKTx in the Toho University single-center study were 13.2±4.0 years and 42.5±6.4 years, respectively. The recipients included 10 boys (48%) and 11 girls (52%). In total, 21 (100%) parents acted as donors for the recipients.
ABO blood group-incompatible matchesIn the Japanese registry of ABOiLDKTx, ABO blood group-incompatible matches included 41 A to O, 16 B to O, 13 AB to A, 8 AB to B, 6 A to B, and 5 B to A. The ABO blood group-incompatible matches in the Toho University single-center study included 6 A to O, 5 B to O, 4 AB to A, 3 AB to B, 2 A to B, and 1 B to A.
ImmunosuppressionIn the Japanese registry of ABOiLDKTx, immunosuppression in the induction period involved the use of RIT (n = 17); basiliximab (BXM; n = 51); splenectomy (n = 55); and azathioprine (AZ; n = 38) or MMF (n = 51) and CsA (n = 48) or FK (n = 41). CsA was replaced with FK in 9 recipients during the maintenance period. Immunosuppression in the induction period in the Toho University single-center study involved the use of RIT (n = 7); BXM (n = 12); splenectomy (n = 14); and AZ (n = 8) or MMF (n = 13) and CsA (n = 11) or FK (n = 10). CsA was replaced with FK in 1 recipient after treatment for acute rejection, and FK was replaced with CsA in 1 recipient who developed post-transplant diabetes mellitus.
The 21 recipients at Toho University were divided into two groups based on immunosuppression. Fourteen recipients who were not administered RIT with splenectomy were categorized into group I. Seven recipients who were administered RIT without splenectomy were categorized into group II.
PreconditioningADBGAB was removed by double-filtration plasmapheresis and plasma exchange using AB blood-group plasma. The ADBGAB titer was adjusted to ≤32 times before ABOiLDKTx. AZ or MMF and steroid were administered for desensitization beginning 10–30 days before ABOiLDKTx. RIT (100 mg) was administered 1–2 times 1 day and/or 14 days before ABOiLDKTx.
Post-transplant conditioningPlasmapheresis, plasma exchange, and intravenous immunoglobulin (IVIG) administration were not performed post-transplantation.
Anti-donor blood-group antibodyADBGAB titers were measured before and after transplantation. Maximum ADBGAB values before and after ABOiLDKTx were compared between groups I and II.
StatisticsPatient and graft survival rates were calculated by the Kaplan-Meier method. A p-value <0.05 was considered significant.
RESULTSPediatric ABOiLDKTx registry under the ABO-Incompatible Transplantation Committee- a.
Number of pediatric ABOiLDKTx cases in Japan
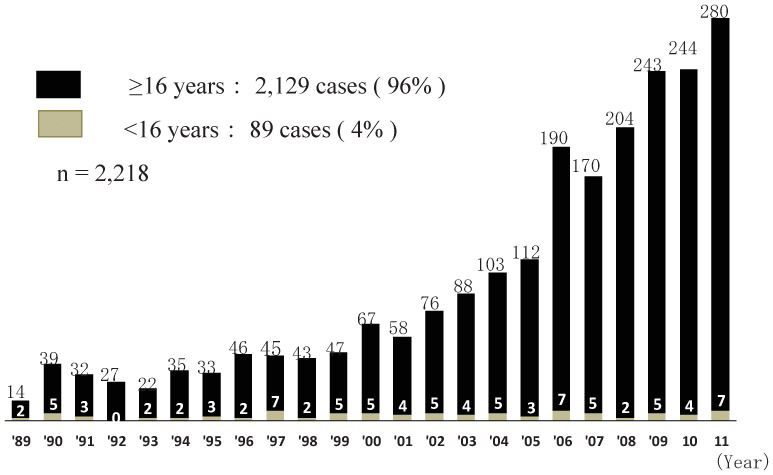
A total of 2,218 ABOiLDKTx procedures were conducted in Japan from 1989–2011. The number of ABOiLDKTx cases has increased annually, and 280 ABOiLDKTx procedures were performed in 2011. Among the 2,218 recipients of ABOiLDKTx, 89 children aged <16 years had undergone ABOiLDKTx (Figure1). Therefore, the rate of pediatric ABOiLDKTx was only 4%, and 0–7 children underwent ABOiLDKTx every year in Japan (Figure1).
- a.
Patient and graft survival rates
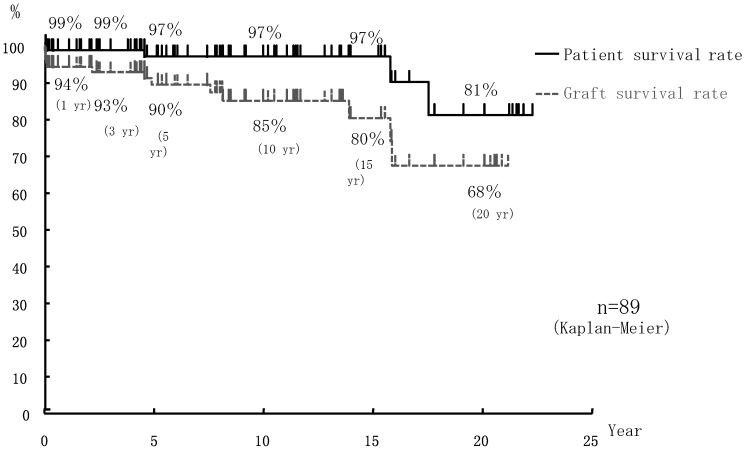
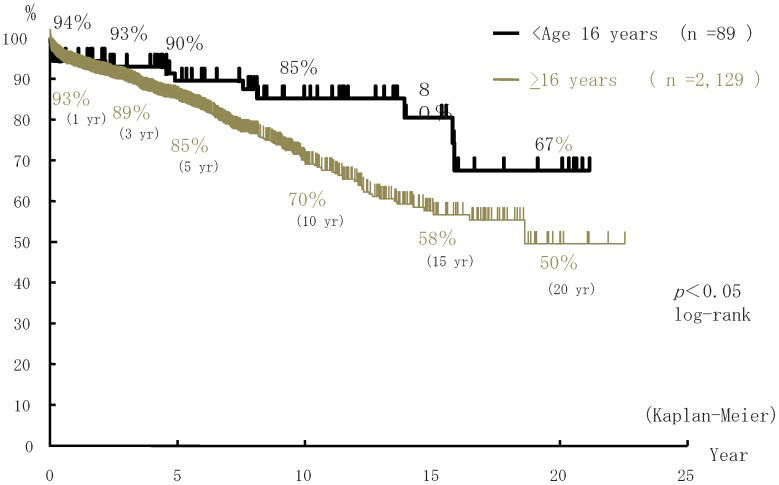
Overall patient and graft survival rates in the 89 pediatric cases of ABOiLDKTx (aged <16 years) were, respectively, 99% and 94% at 1 year, 99% and 93% at 3 years, 97% and 90% at 5 years, 97% and 80% at 10 years, and 81% and 68% at 20 years post-transplantation (Figure2). In contrast, graft survival rates in 2,129 adult cases of ABOiLDKTx (≥16 years) were 93% at 1 year, 89% at 3 years, 85% at 5 years, 70% at 10 years, and 50% at 20 years post-transplantation. Graft survival rates were significantly better in the pediatric cases of ABOiLDKTx than in the adult cases of ABOiLDKTx (p<0.05; Figure3).
Graft survival rates in pediatric recipients of ABOiLDKTx who received CsA (n = 39) or FK (n = 50) were, respectively, 95% and 94% at 1 year, 95% and 91% at 3 years, 92% and 88% at 5 years, 88% and 81% at 10 years, and 68% and 81% at 20 years post-transplantation. No significant difference in graft survival rates was observed between pediatric recipients of ABOiLDKTx who were administered CsA and those who received FK.
Graft survival rates in pediatric recipients of ABOiLDKTx administered RIT without splenectomy (n = 17) and those not administered RIT with splenectomy (n = 18) were 93% and 100% at 1 and 3 years post-transplantation, respectively.
Toho University single-center study- a.
Acute rejection
Acute rejection developed in 8 of 21 recipients. Borderline rejection, grade 1a rejection, and grade 1b rejection, based on the Banff classification, were diagnosed in 3, 4, and 1 recipients, respectively. Eight recipients were administered 250–500 mg steroid pulse therapy. Deoxyspergualin and muromonab-CD3 were administered for steroid-resistant rejection in 3 and 1 recipients, respectively.
Acute rejection occurred in 7 of 14 recipients who were not administered RIT. In contrast, only 1 of 7 recipients (14%) who was administered RIT developed acute grade 1a rejection. No patient who was administered RIT developed steroid-resistant rejection that was not treated with deoxyspergualin and muromonab-CD3.
- a.
Infection
Eight recipients developed a cytomegalovirus (CMV) infection diagnosed by antigenemia. Five of 8 recipients had symptoms of CMV infection, such as fever, including one with CMV pneumonitis. Eight recipients were administered ganciclovir. One recipient had herpes zoster in the right foot and was administered acyclovir. Hemorrhagic cystitis due to adenovirus occurred in one recipient and was cured by MMF withdrawal. Two recipients had wound infections, and one developed a pelvic abscess. The bacterial infections in these two recipients were treated by drainage of pus and antibiotic therapy.
- a.
Other complications
Lymphocele, ureteral stenosis, and B cell lymphoma occurred in one recipient each.
- a.
Patient and graft survival rates (Figure4)
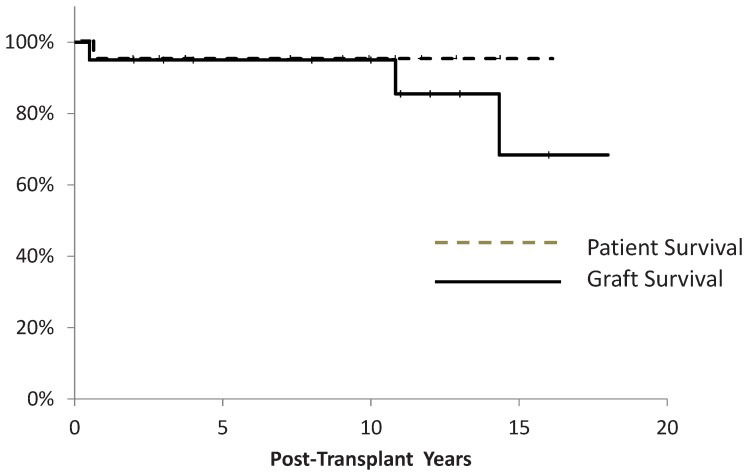
One recipient died of obstructive cardiomyopathy with a functioning graft 6 months post-Tx. The remaining 20 recipients survived for a minimum of 13 months to a maximum of 20 years post-Tx. The patient survival rate was 95% at 10 years post-Tx. Two recipients lost graft function and were reintroduced to hemodialysis 13 years or 10 years and 10 months post-Tx, respectively. Patient and graft survival rates were 95% at 1–10 years post-Tx. One of the two recipients who lost graft function had donor-specific HLA antibodies, and a biopsy demonstrated chronic antibody-mediated rejection. Another recipient developed chronic allograft nephropathy without donor-specific HLA antibodies.
- a.
Sequential change in ADBGAB IgG titers
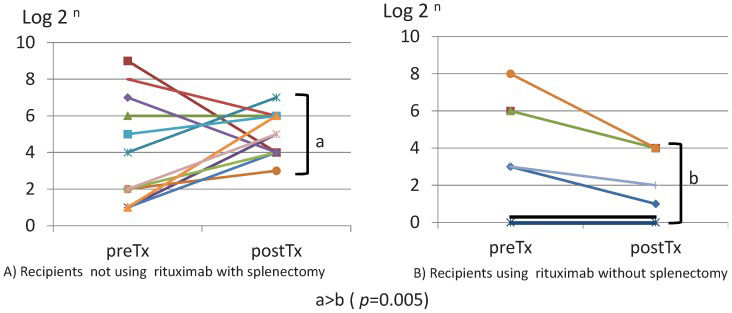
The maximum ADBGAB IgG titers before ABOiLDKTx were 75.9±138.9 and 57.4±85.2 in groups I and II, respectively (p = 0.38; Figure5A). The ADBGAB IgG titers in all 21 recipients immediately before ABOiLDKTx were equal to or less than 32 times in terms of preconditioning. The maximum ADBGAB IgG titers after ABOiLDKTx were 44.0±31.5 and 7.7±7.2 in groups I and II, respectively (p = 0.005; Figure5B). The maximum ADBGAB IgG titers post-transplantation were significantly higher than the maximum IgG titers pre-transplantation in 12 of 14 recipients. However, no recipients belonged to group II.
DISCUSSIONABOiLDKTx has been performed in Japan since 1989. The number of ABOiLDKTx cases has increased annually since 2001, and 280 ABOiLDKTx procedures were performed in 2011. ABOiLDKTx was introduced in Japan because of an extreme shortage of deceased donors and an unexpectedly low incidence of hyperacute rejection (3). In addition, patient and graft survival from ABOiLDKTx have been similar to survival from ABOc since 2001 (2,3). This result may have been caused by the administration of RIT and MMF as desensitizing agents pre-Tx at most kidney transplant centers in Japan since 2000 (4).
ABOiLDKTx has also been performed in children with end-stage renal failure since 1989. In fact, 2 children underwent ABOiLDKTx in 1989 (Figure1). Overall, the graft survival rate in pediatric ABOiLDKTx was better than in adult ABOiLDKTx. More than 90% of the donors in pediatric ABOiLDKTx were parents. The donor age in pediatric ABOiLDKTx was younger than in adult ABOiLDKTx. It is well known that graft survival in KTx depends on donor age. A younger donor provides longer graft survival. No difference in graft survival rates was observed between pediatric ABOiLDKTx recipients administered CsA and those administered FK. FK tended to be used for immunologically high-risk patients, such as HLA-sensitized recipients. However, the immunological risk in ABOiLDKTx recipients could be lower than in HLA-sensitized recipients. Antibody-mediated rejection is avoidfoed because antibody production is directly suppressed by RIT and MMF and is also indirectly suppressed by CNI. According to the NAPRTCS 2010 Annual Transplant Report (5), the graft survival rates in pediatric LDKTx recipients aged <20 years are 95.5% at 1 year, 91.3% at 3 years, 85.7% at 5 years, and 80.5% at 7 years post-Tx. In the Japanese ABOiLDKTx registry, graft survival rates in children aged <16 years were 94% at 1 year, 93% at 3 year, and 90% at 5 years post-Tx. Although the definition of the age of a child is different between the NAPRTCS (age <20 years) and the Japanese registry of pediatric ABOiLDKTx (age <16 years), graft survival rates were not different. However, the definition of the age of a child at Toho University was the same as that provided by the NAPRTCS. The graft survival rate in pediatric ABOiLDKTx at Toho University was better than that reported by the NAPRTCS. However, the number of ABOiLDKTx procedures at Toho University was small, and most of the NAPRTCS data included pediatric ABOcLDKTx. The incidences of acute rejection and antibody-mediated rejection in a Japanese study comparing pediatric ABOc and ABOiLDKTx were significantly higher in ABOiLDKTx than in ABOc. However, no difference in patient and graft survival rates was observed between the groups (97%, 92%, 88%, and 86%, respectively, at 10 years post-transplantation).
It appeared that there was a lower incidence of antibody-mediated rejection in pediatric recipients of ABOiLDKTx who were administered RIT without splenectomy, and no patient developed steroid-resistant antibody-mediated rejection at Toho University. The graft survival rate in recipients administered RIT without splenectomy was not different from that in recipients not administered RIT with splenectomy in the Japanese registry of pediatric ABOiLDKTx. Recipients who were administered RIT without splenectomy at Toho University tended to have a lower incidence of acute rejection, although the difference was not significant. In addition, the recipients who were administered RIT without splenectomy showed lower ADBGAB IgG titers post-Tx than those not administered RIT with splenectomy. ABOiLDKTx recipients not administered RIT with splenectomy tended to have increased ADBGAB titers that were greater than the maximum pre-Tx titer within 10 days (6). RIT appeared to be a stronger suppressor of antibody production than splenectomy was. Splenectomy is not necessary to avoid hyperacute rejection and antibody-mediated rejection in patients undergoing pediatric ABOiLDKTx if RIT is administered as a desensitizing agent pre-transplantation (7). A splenectomy should be avoided in children to prevent pneumococcal infection and other complications, although our 14 pediatric recipients who underwent splenectomy at Toho University had no serious complications.
The incidence of acute rejection in pediatric recipients administered RIT without splenectomy tended to be lower than in those not administered RIT with splenectomy in the Toho University single-center study. In particular, steroid-resistant rejection did not occur in any pediatric recipients administered RIT without splenectomy.
The maximum ADBGAB IgG titers post-transplantation in group II (pediatric recipients of ABOiLDKTx who were administered RIT without splenectomy) were significantly lower than those in group I (who were not administered RIT without splenectomy). The ADBGAB IgG titers increased in 12 of 14 recipients in group I post-Tx; however, the titers in group II did not increase. Post-transplant plasmapheresis or adsorption is routinely performed in European and US reports (7,8). Therefore, these reports cannot show the natural course of the ADBGAB titers post-Tx. In contrast, we did not perform post-transplant plasmapheresis or exchange at Toho University. Therefore, we were able to analyze the sequential changes in ADBGAB titers during immunosuppression. Takahashi reported that antibody-mediated rejection related to ABO blood-group incompatibility does not occur due to natural ADBGAB, but rather to de novo ADBGAB (9). Desensitization with RIT, MMF, and CNI pre-Tx and immunosuppression of B cells post-Tx in ABOiLDKTx recipients are more important than plasmapheresis pre- and post-Tx. The Japanese ABO-Incompatible Transplantation Committee recently recommended that plasmapheresis or exchange should not be performed as preconditioning if the ADBGAB IgG titer is ≤64 times higher in recipients and stated that routine plasmapheresis is not necessary post-Tx. These recommendations are beneficial for small children with peritoneal dialysis because they do not require blood access and a catheter for plasmapheresis or exchange.
ACKNOWLEDGMENTSWe appreciate the ABO-Incompatible Transplantation Committee members who supplied data for the Japanese registry of pediatric ABOiLDKTx.
AUTHOR CONTRIBUTIONSAikawa A is the corresponding author; he collected and analyzed the data of ABO-Incompatible Pediatric Kidney Transplantation in Toho University, summarized the Japanese registry of ABO-Incompatible Pediatric Kidney Transplantation and wrote the manuscript. Kawamura T, Shishido S and Aikawa A work at the Clinic of ABO-Incompatible Pediatric Kidney Transplantation at Toho University; they partially revised the manuscript. Saito K and Takahashi K conceived the Japanese registry of ABO-Incompatible Pediatric Kidney Transplantation and participated in manuscript design, coordination, drafting and revision. Takahashi K, Saito K and Shishido S participated in the final revision of the manuscript.
No potential conflict of interest was reported.