This study aimed to characterize and estimate the frequency of adverse reactions to antituberculosis drugs in the population treated at the Centro de Saúde Escola Germano Sinval Faria, a primary health care clinic in Manguinhos, Rio de Janeiro City, and to explore the relationship between adverse drug reactions and some of the patients' demographic and health characteristics.
METHODSThis descriptive study was conducted via patient record review of incident cases between 2004 and 2008.
RESULTSOf the 176 patients studied, 41.5% developed one or more adverse reactions to antituberculosis drugs, totaling 126 occurrences. The rate of adverse reactions to antituberculosis drugs was higher among women, patients aged 50 years or older, those with four or more comorbidities, and those who used five or more drugs. Of the total reactions, 71.4% were mild. The organ systems most affected were as follows: the gastrointestinal tract (29.4%), the skin and appendages (21.4%), and the central and peripheral nervous systems (14.3%). Of the patients who experienced adverse reactions to antituberculosis drugs, 65.8% received no drug treatment for their adverse reactions, and 4.1% had one of the antituberculosis drugs suspended because of adverse reactions. “Probable reactions” (75%) predominated over “possible reactions” (24%). In the study sample, 64.3% of the reactions occurred during the first two months of treatment, and most (92.6%) of the reactions were ascribed to the combination of rifampicin + isoniazid + pyrazinamide (Regimen I). A high dropout rate from tuberculosis treatment (24.4%) was also observed.
CONCLUSIONThis study suggests a high rate of adverse reactions to antituberculosis drugs.
Tuberculosis is the chronic infectious disease that kills the most individuals worldwide. Since 1993, the World Health Organization (WHO) (1,2) has considered tuberculosis a global emergency. In 2005, there were an estimated eight million new cases and approximately two million deaths per year worldwide, with only half the cases reported to the WHO (1). The main factors aggravating the situation are social inequality, the spread of AIDS, an aging population, major migratory movements (3), improper health care (4), and a lack of information, which is associated with these previous factors and one of the key obstacles to controlling the disease (2).
In Brazil, “…tuberculosis is neither an emerging nor a re-emerging public health problem. It is a problem that has been present and resident for a long time” (3). Brazil ranks 19th among countries with the most tuberculosis cases in the world today (5). Nationwide, some 5,000 to 6,000 deaths from tuberculosis are recorded annually (6). Recently, the number of new cases in Brazil has declined significantly, from 51.44/100,000 in 1999 to 37.12/100,000 in 2008 (7). Nonetheless, in 2008, incidence in the Rio de Janeiro municipal area was 68.64/100,000 (8), the highest in the country, while in some of the municipality's most underserved areas, such as the Manguinhos district, annual incidence reached 145/100,000 (9).
Manguinhos has Rio de Janeiro's third-lowest Human Development Index, is considered the city's worst district in terms of basic sanitation, and houses a large number of former prison inmates. Its population profile features a high proportion of chemical- and alcohol-dependent individuals and a large street population that is characterized by extreme poverty and little schooling. These problems accentuate the difficulties of controlling tuberculosis (10).
The Ministry of Health (MoH) considers that compliance with the appropriate recommended therapeutic regimen makes tuberculosis curable in essentially 100% of cases. Using antituberculosis drugs correctly for an adequate period ensures a cure (6).
Although they are effective at combating Mycobacterium tuberculosis, tuberculosis drugs can cause adverse reactions that may jeopardize treatment and contribute to nonadherence (11).
An adverse drug reaction (ADR) consists of “a response to a drug that is noxious and unintended and occurs at doses normally used in man for the prophylaxis, diagnosis, or therapy of disease” (12).
Treatment dropout impairs control of the disease, hinders its cure, and increases treatment time and costs (4).
In Manguinhos and other communities in major urban centers, the tuberculosis incidence is much higher than the national and local means; accordingly, this higher incidence deserves detailed study. This study aimed to characterize and estimate the frequency of adverse reactions to antituberculosis drugs in the population treated at the Centro de Saúde Escola Germano Sinval Faria (CSEGSF), a primary health care clinic in Manguinhos, Rio de Janeiro City. In addition, the study explored the relationship between adverse drug reactions and some of the patients' demographic and health characteristics.
METHODSThis study forms part of the project “Fatores associados aos desfechos do tratamento da tuberculose em um centro de atenção básica de saúde: uma análise sob a ótica antropológica e epidemiológica” (13) [Factors associated with tuberculosis treatment outcomes at a primary health care clinic: an anthropological and epidemiological analysis], which is financed by the Global Fund to Fight Tuberculosis, Aids and Malaria and conducted at CSEGSF/ENSP/Fiocruz/RJ.
Information was drawn from the Rio de Janeiro compulsory notifications database (SINAN/SMS RJ), patient records, the CSEGSF case log book, and the computerized patient register. These sources served as the basis for project researchers (13) to define the tuberculosis “cases” used for the study of adverse reactions.
Study design and populationThis study was descriptive via retrospective review of patient medical records.
The population consisted of new pulmonary tuberculosis cases treated at the CSEGSF outpatient department. According to the MoH Manual Técnico para o Controle da Tuberculosis, a case of tuberculosis is any individual with a diagnosis confirmed by sputum smear test or culture and for whom the physician establishes a diagnosis of tuberculosis based on clinical and epidemiological data and the results of complementary examinations. A new case is someone with tuberculosis who has never undergone antituberculosis chemotherapy, has done so for less than 30 days, or was treated for tuberculosis more than five years ago (14).
Included in this study were children, adults, and older adults of both sexes who were residing in the Manguinhos district of Rio de Janeiro, diagnosed with pulmonary tuberculosis, and considered new cases between January 2004 and December 2008. During the study, 291 cases of tuberculosis were identified in the SINAN, and 370 cases were identified in the case log book. After matching the datasets and excluding duplicate records and cases that were reported more than once because of relapse or return after dropout, 279 cases remained for patient record analysis. After the inclusion criteria were applied, 176 cases remained eligible for the study. The records of 103 patients were excluded for the following reasons: not being considered new cases of pulmonary tuberculosis (relapse or return after dropout, n = 25); residency in other districts of Rio de Janeiro (n = 14); other types of tuberculosis (n = 12); not having completed at least 30 days of treatment (n = 13); transfer for follow-up to another clinic (n = 13); changes of diagnosis (n = 20); and incomplete records that made it impossible to identify the closure status (n = 6; Figure 1.
Patients were followed up until case closure by cure, dropout, failure/multidrug-resistant tuberculosis (MDR-TB), or death.
Information sources, data collection, and study variablesFor data collection, a patient record data collection form and a form-completion instructions manual were developed in an electronic format using Access (v. 2.0, 2003). The two instruments were standardized and tested.
The information drawn from the patient records was complemented with project sources (13).
Data were collected by the lead researcher in June and July 2010. To ensure quality at this stage, two other authors (LG and SR) collected data again from 10% of the patient records. At this stage, no major divergences were found. Some aspects of data recording that had no impact on the estimates were standardized.
The following variables were considered:
Adverse drug reaction (ADR): Information drawn from the progress sheets in patients' records, with the respective dates.
Case closure status: Information drawn from data collected for the project (13). The variable categories and definitions (6) were as follows:
Dropout: “Any patient who fails to attend the clinic for more than 30 consecutive days after the scheduled return date. In cases of supervised treatment, the 30-day limit is counted from the last drug administration.”
Failure/MDR-TB: “Persistently positive sputum smear tests at the end of treatment. Also classified as 'Failure’ are patients who are strongly positive (++ or +++) at start of treatment and maintain that status until the fourth month, as well as all those who start treatment positive, then are negative, then once again positive for two consecutive months, as of the fourth month of treatment.”
Death: “Classified upon learning of the patient's death during treatment, regardless of cause.”
Cure: “Cases initially positive for pulmonary TB: discharge by cure will be given when, on completing treatment, the patient returns two negative sputum smear tests, one during the follow-up stage and one upon completion of treatment (cure). Discharge by cure will also be given when treatment is deemed complete based on clinical and radiological criteria and complementary examinations.”
Associated comorbidities: Information drawn from patient records. All comorbidities were recorded for subsequent analysis using the Naranjo algorithm (15), a tool for evaluating the causal relationship between drug use and adverse reaction. The date of occurrence was that of the first recording of each comorbidity during the patient's treatment period. The International Classification of Diseases (ICD-10) was attached to the electronic form so that the ICD codes described in the record form could be identified and the morbidities described in the record form could be assigned an ICD code. All prior diseases, such as hypertension, diabetes, AIDS, and alcoholism, recorded by the medical team were considered. Any symptoms or signs that might be ADRs were also recorded.
Sex and age: information was drawn from patient records. Age was recorded as a continuous variable and subsequently categorized into the following age groups: 0 to 19 years, 20 to 49 years, and 50 years or older.
Drugs used: Information was drawn from patient record progress sheets, with prescription dates. Antituberculosis drugs (rifampicin, isoniazid, pyrazinamide) and other drugs, both continuous- and occasional-use, were considered. The following categories were created for the antituberculosis drugs: prescribed (P), suspended by patient (SP), suspended by medical practitioner (SM), dropout (D), completion of therapy (C), and non-protocol doses (P*). For the analysis of the total number of antituberculosis and other drugs used, two categories were created: up to four and five or more. Drugs used during treatment were counted, and repeat prescriptions were disregarded.
Data analysisTwo components were considered in the analysis: patients and ADRs. The data on patients and ADRs are given in absolute values and as proportions. The mean and median behavior of the continuous variables was also noted. Proportions were compared using the Pearson chi-squared (χ2) test.
Patients were classified by occurrence of ADR with the aid of the Naranjo algorithm, which contains ten questions, each of which receives a score. The questions specifically address the compatibility between when the reaction appeared and when the drug was taken; the nature of the reaction; the drug's pharmacological characteristics; and alternative causes (15). The algorithm enables ADRs to be classified as “definite”, “probable”, “possible”, or “doubtful”. In the case of doubtful reactions, patients were classified as not experiencing an ADR.
Recording the dates of interest (prescription of each drug; appearance of the ADR; comorbidities identified) made it possible to rigorously identify the time relation between prior exposure to the drugs and the subsequent appearance of adverse reactions.
ADRs were classified by the organ system affected according to WHO terminology (WHO-ART, 2005) (16) and described by type, period of occurrence, and severity.
Severity classifications were based on the MoH definitions (6), as follows: mild effects, in which there is no immediate modification of the standard regimen, and major effects, which may entail interruption or alteration of the treatment. The identification of ADRs not described by MoH was based on the scientific literature (17,18,19,20).
To explore the relationships between the ADRs and the characteristics of the study population, a multiple correspondence analysis (MCA) was used. This is a descriptive or exploratory multivariate technique.
The analysis was essentially graphic and was conducted by observing proximity among points in a multidimensional space representing the spatial configuration of the categories of variables analyzed (21).
The analyses were performed using the statistical package SPSS for Windows, Version 17.0. Correspondence analysis was performed using the software XLSTAT 4.0.
Ethical considerationsThe project was approved by the Oswaldo Cruz Foundation (CEP/ENSP/Fiocruz) ethics committee for research with human subjects under Number 0038.0.031.000-09.
RESULTSThe 176 patients selected were included in the analyses. Their ages ranged from 1 to 85 years (mean = 32.4 years; median = 29 years). Of the participants, 113 (64.2%) were male, 128 (72.7%) were 20 to 49 years old, 161 (91.5%) had up to three recorded comorbidities, and 117 (66.5%) used up to four drugs (Table 1). Of the total patient population, 126 (71.6%) were cured, while 43 (24.4%) dropped out of treatment, five (2.8%) died from tuberculosis, one (0.6%) died from other causes, and one (0.6%) had multidrug-resistant tuberculosis. The mean number of drugs used was 4.46 (median = 3).
Population characteristics of new tuberculosis cases by occurrence of adverse reactions (ADRs) to antituberculosis drugs, CSEGSF/ENSP/FIOCRUZ-RJ, 2004-2008.
| Patient Characteristics | Without ADRs | With ADRs | Total |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Gender | |||
| Male | 74 (65.5) | 39 (34.5) | 113 (64.2) |
| Female*) | 29 (46.0) | 34 (54.0) | 63 (35.8) |
| Age group | |||
| 0 to 19 years | 21 (77.8) | 6 (22.2) | 27 (15.3) |
| 20 to 49 years *) | 72 (56.2) | 56 (43.8) | 128 (72.7) |
| 50 years or older *) | 10 (47.6) | 11 (52.4) | 21 (12.0) |
| Comorbidities # | |||
| None | 50 (79.4) | 13 (20.6) | 63 (35.8) |
| 1 to 3**) | 50 (51.0) | 48 (49.0) | 98 (55.7) |
| 4 or more**) | 3 (20.0) | 12 (80.0) | 15 (8.5) |
| Number of drugs | |||
| 0 to 4 | 80 (68.4) | 37 (31.6) | 117 (66.5) |
| 5 or more**) | 23 (39.0) | 36 (61.0) | 59 (33.5) |
| Total | 103 (58.5) | 73 (41.5) | 176 (100.0) |
The most frequent comorbidities among the patients studied were lifestyle-related problems (tobacco and/or alcohol; 23.4%), hypertension (12.6%), diabetes (11.7%), and HIV (10.8%).
In the study sample, the proportion of ADRs among women was greater than among men (54.0% vs. 34.5%). Patients aged 50 years or older displayed a higher proportion of ADRs than other age groups (52.4% vs. 22.2% among the 0- to 19-year-old subjects). The stratum with four or more comorbidities showed a higher proportion of ADRs than others (80.0% vs. 20.6% for no comorbidities). The stratum using five or more drugs showed a higher proportion of ADRs (61% vs. 31.6% among users of up to four drugs; Table 1).
Of the 176 patients studied, 73 (41.5%) developed one or more ADRs, for a total of 126 occurrences (Tables 1) and 2). Of the 73 patients with ADRs, 39 developed only one ADR, while 34 patients developed two or more ADRs during the study period.
Frequency of adverse drug reactions (ADRs) to antituberculosis drugs by type and severity, CSEGSF/ENSP/FIOCRUZ-RJ, 2004-2008.
| Description of adverse reactions | N | (%) |
|---|---|---|
| Minor adverse reactions | 90 | 71.4 |
| Gastric irritation (nausea, vomiting), abdominal pain | 37 | 29.3 |
| Itchy skin | 20 | 15.9 |
| Arthralgia or arthritis | 13 | 10.3 |
| Headache | 6 | 4.8 |
| Sweat and urine orange | 6 | 4.8 |
| Behavior change (euphoria, insomnia, anxiety, and drowsiness) | 4 | 3.1 |
| Peripheral neuropathy (burning of the extremities) | 3 | 2.4 |
| Fever | 1 | 0.8 |
| Major adverse reactions | 8 | 6.4 |
| Exanthema | 3 | 2.4 |
| Hepatotoxicity (vomiting, hepatitis, changes in liver function tests) | 3 | 2.4 |
| Thrombocytopenia, leukopenia, eosinophilia, hemolytic anemia, agranulocytosis, vasculitis | 2 | 1.6 |
| Other§) | 28 | 22.2 |
| Total | 126 | 100.0 |
ADRs not described in the MoH Guia de Vigilância Epidemiológica: adenopathy, hallucinations, amenorrhea, anemia, increased uric acid, increased menstrual flow, painful urination, rash, weakness, jaundice, desquamative (scaly) lesions on the knees, urticarial lesions, malaise (sickness), other psychotic disorders, allergic reactions, and dizziness.
Approximately 78% (98/126) of the ADRs are described in the MoH epidemiological surveillance guide (Guia de Vigilância Epidemiológica) (6). Of the ADRs found in this study, 71.4% were considered mild reactions, 6.4% were major, and 22.2% were considered “other”. Of the latter, the most frequent (32.1%) was dizziness (Table 2). No death from antituberculosis ADR was observed.
Of the total patients with ADRs, 48 (65.8%) received no drug treatment for the ADR, and three (4.1%) had one of the antituberculosis drugs suspended because of an adverse reaction.
The organ systems most affected by ADRs were the gastrointestinal tract (29.4%), the skin and appendages (21.4%), the central and peripheral nervous systems (14.3%), and the musculoskeletal system (10.3%). Disturbances of the urinary system, the liver and gall bladder, the female reproductive system, the white and red blood cells and reticuloendothelial system, metabolism and nutrition, and the overall state of health and psychiatric disturbances were also found. Each of these disturbance types comprised between 0.8 and 5.5% of the cases analyzed.
Table 3) describes the ADRs according to the treatment period during which they occurred and according to the severity of the ADR and the related drugs. ADRs may be associated with more than one drug, which share an array of undesirable side-effects, attributable in part to common pharmacokinetic processes. In the study sample, 81 (64.3%) of the reactions occurred during the first two months of treatment, and most reactions (92.6%) were imputed to the combination of rifampicin + isoniazid + pyrazinamide (Regimen I). The severest ADRs tended to occur from the third month of treatment onwards (p<0.05).
Adverse drug reactions (ADRs) to antituberculosis drugs by severity, drugs involved, and treatment period, CSEGSF/ENSP/FIOCRUZ-RJ, 2004-2008.
| Up to 2nd month N (%) | 3rd month onwards N (%) | Total ADRs N (%) | |
|---|---|---|---|
| Type of ADR#*) | |||
| Minor | 64 (71.1) | 26 (28.9) | 90 (91.8) |
| Major | 3 (37.5) | 5 (62.5) *) | 8 (8.2) |
| Drugs | |||
| R+H+Z | 50 (92.6) | 4 (0.8) | 54 (42.9) |
| R+H | 16 (42.1) | 22 (57.9) | 38 (30.2) |
| R | 7 (50.0) | 7 (50.0) | 14 (11.1) |
| H | 3 (33.3) | 6 (66.7) | 9 (7.1) |
| R+Z | 2 (100.0) | - | 2 (1.59) |
| Z | - | 1 (100.0) | 1 (0.8) |
| R+E | 1 (100.0) | - | 1 (0.8) |
| R+H+E | - | 1 (100.0) | 1 (0.8) |
| R+H+Z+E | - | 1 (100.0) | 1 (0.8) |
| Other combinations§) | 2 (40.0) | 3 (60.0) | 5 (4.0) |
| Total | 81 (64.3) | 45 (35.7) | 126 (100.0) |
R = rifampicin; H = isoniazid; Z = pyrazinamide; E = ethambutol.
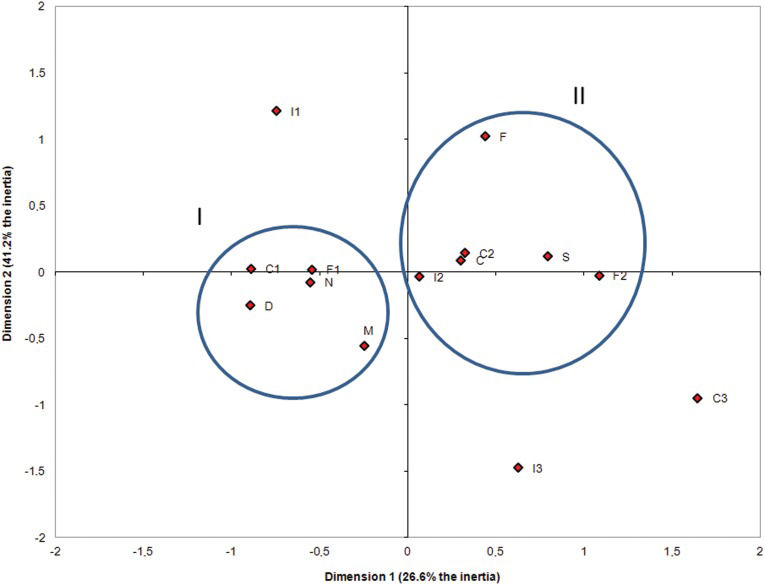
D – dropout; C – cure; N – no ADR; S – ADR present; F – female; M – male; I1 – aged 0 to 19 years.; I2 – aged 20 to 49 years; I3 – aged 50 or older years; F1 – used up to four drugs; F2 – used five or more drugs; C1 – no comorbidity; C2 – one to three comorbidities; C3 – more than three comorbidities.
Twenty-eight ADRs were excluded because they are not described in the MoH Guia de Vigilância Epidemiológica. Half of the ADRs occurred during the first two months of treatment.
Other combinations include: rifampicin + ethinylestradiol; rifampicin + isoniazid + paracetamol + sulfadiazine + sulfamethoxazole + omeprazole; rifampicin + efavirenz + metronidazole + sulfamethoxazole; rifampicin + isoniazid + efavirenz + sulfadiazine + sulfamethoxazole + pyrimethamine; isoniazid with alcohol intake.
The likelihood of an adverse reaction being caused by the drugs was analyzed using the Naranjo algorithm. The results showed a predominance of probable reactions (75%), followed by possible reactions (24%) and definite reactions (1%).
A high rate of dropout from TB treatment (24.4%) was observed (43/176). The dropout rate was higher among the men than women (29.4% vs. 18.3%) and higher among younger people (37% were from 0 to 19 years old vs. 23.6% in the 20 to 49 years group and 21.1% in the 50 years or older group), although the differences were not statistically significant. Dropout was also observed to be greater (p<0.05) among those with no recorded comorbidities (38.7% vs. 18.5% with one to three comorbidities and 13.3% with four or more comorbidities), those who used up to four drugs (32.7% vs. 10.7% of users of five or more drugs), and those who developed no adverse reactions to the antituberculosis drugs (34.0% vs. 13.0% among those with an ADR).
The graph analysis appears to show two groups, each represented by one ellipse. The small ellipse shows the group of individuals who dropped out of treatment, are male, used up to four drugs, had no comorbidities, and did not develop ADRs (A, M, F1, C1, and N). The bigger ellipse shows the group of individuals who were cured, were aged 20 to 49 years, used more than five drugs, presented one to three comorbidities, and developed ADRs (C, I2, F2, C2, and S; Figure 2.
DISCUSSIONThe cases of tuberculosis treated at the CSEGSF/Ensp/Fiocruz were predominantly patients who were men, adults, had up to three comorbidities, used up to four drugs, and evolved to cure.
One of the main findings of this study was that 42% of the patients presented antituberculosis ADRs, three-quarters of which were classified as “probable”. Of the total ADRs, 78% were described in the MoH epidemiological surveillance guide (Guia de Vigilância Epidemiológica), and 71% were considered mild. One-third of the reactions affected the gastrointestinal system, although essentially all systems were affected.
The study showed a high rate of ADRs, similar to that found by a study in São Paulo city (22) (49.1%) among patients treated at a teaching hospital outpatient department. In Canada, a retrospective study of 1,061 patients found a 30% rate of adverse reactions to antituberculosis drugs (23). However, prospective studies in Brazil have shown that the rate adverse reactions to antituberculosis drugs may be still higher, reaching 83.5% of patients (24).
The positive associations between ADR and female sex and advanced age may have differing interpretations, as found in other studies (11,25,26). Regarding female sex, the mechanism is unknown; however, previous interpretations include hormonal oscillations at different stages of life that modify drug responses; interactions between antituberculosis and contraceptive drugs that may favor the appearance of ADRs (17,19); or greater attendance at health services, which would facilitate the detection of ADRs. Regarding age, the older population is more prone to develop ADRs, particularly the most severe forms (11,23,25,26). Patients older than 60 years are likely to experience intoxications from these drugs (14) because both hepatic and renal metabolisms can be impaired with age, favoring the accumulation of metabolites and the occurrence of ADRs (27).
Tuberculosis may be concomitant with other diseases, thus increasing the number of drugs patients use and predisposing them toward adverse reactions. In the study sample, patients who used more than five drugs in the course of their treatment developed more ADRs. Kishore et al. (11) observed in India that nearly half the patients who developed ADRs were prescribed more than five drugs.
In terms of severity, a higher frequency of mild reactions was observed. This is consistent with the findings of a study conducted in São Paulo city, in which 81% of the ADRs were considered mild, and the most common ADRs were abdominal pain (20.4%) and arthralgia (16.4%) (22).
Regarding the nature of the ADRs, gastrointestinal disturbances, skin conditions, and joint pains were the most commonly identified problems. Gastrointestinal disturbances, which are characterized by gastric irritation, nausea, vomiting, and abdominal pain, were present in the epidemiological studies (11,22,23,25) at frequencies ranging from 14.28% (11) to 29.7% (22). It is particularly important to note reactions that impair the liver because they may require interrupting treatment and may even lead to death. In a national study of 297 patients in São Paulo, 3.7% of the patients (n = 11) had their drug regimen altered because of adverse reactions, and hepatotoxicity was responsible for 63.7% of the changes (22). Compared with other studies (11,22,23,25), our sample showed a low percentage of hepatotoxicity (2.4%), which may be explained in part by the fact that complementary examinations to detect evidence of hepatic alterations could not be performed on the 43 patients (24.4%) who dropped out of treatment.
Only 30.1% of the antituberculosis ADRs were treated at CSEGSF. ADRs must be identified and treated to prevent similar reactions, reduce health service costs, and minimize patient suffering.
ADRs are known to occur predominantly in the early months of tuberculosis treatment (22,25,26,28), although severe effects, such as hepatotoxicity, may occur later, after the fifth month of treatment (29). Similarly, in our study, most (64.3%) of the ADRs occurred in the first two months of treatment, which may be explained by the fact that the three drugs associated with most of the ADRs were used simultaneously during that period.
Establishing the probability that the relationship between drug and ADR is causal (causality) depends largely on the information obtained, particularly regarding alternative causes, such as other diseases and the use of other drugs. Our findings permit most (75%) of the adverse reactions to be characterized as probably occasioned by the drugs. A retrospective study of 326 patients in India found an estimate similar to ours (72.5%) (11). Meanwhile, a retrospective study of 1,061 patients in Canada classified 51.7% as likely and 20.9% as definitely drug-caused (23). Instruments for analyzing causality (30,31) are grounded in basic considerations, such as temporality, pharmacological plausibility, and alternative causes. For that reason, the Naranjo probabilities scale (15), a method that has been previously validated, in widespread use for several decades, and applied in both prospective and retrospective studies, was used in this study.
Even given the use of tools to ensure that causality is judged objectively, the quality of the information obtained in each study and the source of scientific evidence can explain the variations between estimates.
The drugs for tuberculosis are effective and should be used in the correct dosages for a period of six months. One factor of concern is the rate of treatment dropout before the end of that period, which impairs the drugs' effectiveness and can lead to multiple-drug resistance. For that reason, we were surprised at the percentage of patients who dropped out of treatment (24.4%). In 2001, the national mean dropout rate was approximately 12% (32), and it reached 27.3% in some state capitals (33). The WHO target is to reduce that rate to 5% (34). The high dropout rate reflects the tuberculosis scenario in many localities in Brazil, where social problems such as hunger, extreme poverty, violence, and drug abuse are themselves risk factors for treatment dropout [36]. It is thus possible that for these patients, adherence to tuberculosis treatment involves other issues that require more complex, systemic solutions.
Losses from patients who dropped out of treatment between January 2004 and December 2008 prevented an analysis of the association between dropout and ADRs. To explore the hypothesis that the adverse effects of the treatment may lead to dropout would entail actively finding the cases that failed to attend the clinic. That would be extremely difficult in the context of this study, in which the time lag would make it difficult to trace losses. Alternatively, approaches could be developed to include prospective follow-up of the participants, as will be discussed below.
From the correspondence analysis, it was possible to identify two groups. The first comprised predominantly men, younger patients, those with fewer comorbidities and adverse reactions, those using fewer drugs, and those who dropped out of treatment. Interestingly, higher dropout rates among men and among young patients were also observed in another retrospective study of 481 smear-positive patients conducted in Cuiabá (Mato Grosso) from 1998 to 2000 (25). The second group included predominantly women, older patients, those with more comorbidities and adverse reactions, those using more drugs, and those who evolved to cure.
It is plausible that the second group frequented the clinic more assiduously, which would enable the adverse reactions to be more readily identified and recorded. The group comprising mainly men, young patients, and those with fewer comorbidities may have dropped out of treatment; accordingly, fewer adverse reactions were recorded. This latter group may have been more irregular in the treatment (failing to take the drugs for a few days), may have presented milder adverse reactions more often in the first two months, and may not have returned to the clinic for subsequent appointments. Thus, those who dropped out of treatment would have demographic characteristics that would warrant supposing that the ADRs may have been the reason for dropout. There may, however, be other more important reasons, such as the socioeconomic situation itself, in the case of young patients.
Regarding the study's limitations, it should be noted that the retrospective design, handwritten patient records, and patients' failure to attend appointments may all have undermined the credibility of the information on the drugs prescribed, dosages and frequency of drug use by the patients, and the patients' clinical progression. Nonetheless, given the overall quality of the records and the completeness of the information observed during data collection, it can be said that generally, the validity of the records is assured and did not impair the general information about exposure to the drugs. Regarding the information on social variables, such as schooling, little information was entered in the records: no information on level of schooling was available for 71.6% (126/176) of the patients studied.
The information in the clinic's patient records was sufficient for the study of ADRs, and the estimates are compatible with expected values. The fact that the clinic in question engages in technology development, research, and teaching in the health field certainly contributed to our ability to obtain information of acceptable quality and quantity. Electronic patient records and prescriptions and improvements in patient data recording may be introduced in the future and would contribute to improving the quality of research at the clinic.
Furthermore, regarding methodological considerations, it should be noted that the differences in the study designs hindered comparisons of their results. It should also be remembered that in many countries, the main tuberculosis treatment regimen includes ethambutol (which was only recently adopted in Brazil) and streptomycin, which are considered first-line drugs.
For analysis purposes, the information on comorbidities was considered only once, at the date of the first entry in the patient record. Additionally, the comorbidities and ADRs were assumed to be concomitant, regardless of the date when the comorbidities were recorded. That is because the retrospective design and degree of irregularity in attending appointments made it impossible to guarantee whether acute conditions recorded at a time inconsistent with (previous or subsequent to) the occurrence of the ADR actually occurred at the time of the ADR. For chronic conditions, such as hypertension or diabetes, that assumption does not entail errors. For acute, symptomatic conditions, however, comorbidities may have been overestimated, and ADRs may have been underestimated.
A literature search found no studies addressing the association between dropout and the incidence of adverse reactions, in part because observational studies of adverse reactions to tuberculosis treatment are still scarce in Brazil. Future studies should examine that association, although the difficulties of such an examination are not insignificant.
In retrospective studies, such as the present one, testing associations is problematical because the occurrence of ADRs among patients who dropped out of treatment is recorded only for the period during which the patient remains in treatment, and that time varies from individual to individual. In prospective studies, testing the association between adverse reactions and dropout would raise ethical issues and entail modifying the definition of the outcome variable. Here, dropout from treatment was considered to be failure to attend an appointment for 30 days or more, which was based on the MoH criteria. A prospective approach could consider the intention to drop out of treatment or the failure to attend an appointment for a shorter period. In addition, detecting patients who dropped out of treatment could also yield useful information about the reasons for dropout, which may include the occurrence of ADRs.
To conclude, this study suggests a high rate of ADRs, which were detected particularly in one subgroup of patients. Various measures can be taken to reduce those figures, particularly measures to increase awareness among patients and health personnel. Regarding the latter, it would be necessary to introduce changes to the work process and how production is organized, with a view toward spreading technical knowledge about the occurrence of ADRs and establishing mechanisms so that ADRs are appropriately recorded, treated and controlled. From a research standpoint, prospective studies in the population of the Manguinhos district and others with similar social and economic profiles could produce new information to help control adverse reactions and increase adherence to tuberculosis treatment.
AUTHOR CONTRIBUTIONSDamasceno GS was responsible for the literature review, data collection, data analysis, and drafting the manuscript. Guaraldo L participated in the design, planning, collection, analysis, interpretation of data, and critical revision of intellectual content. Engstrom E participated in planning the study. Theme Filha M conducted the field activities. Souza Santos R participated in the planning and preparation of the data entry program. Vasconcelos AG participated in the statistical analysis and interpretation of data. Rozenfeld S was responsible for conceiving and designing the study and participated in the planning, collection, analysis, interpretation of data, and critical revision of intellectual content. All authors read and approved the final manuscript.
We would like to thank the postgraduate program in Epidemiology at the Escola Nacional de Saúde Pública Sérgio Arouca (ENSP/Fiocruz), which made this study based on the main author's MSc thesis possible; the project of the Global Fund to Fight Tuberculosis, Aids and Malaria; and Professor Dora Chor for her critical reading of the manuscript.
No potential conflict of interest was reported.