We evaluated the effects of aerobic exercise training without dietary changes on cardiovascular and metabolic variables and on the expression of glucose transporter Type 4 in rats with metabolic syndrome.
METHODSTwenty male spontaneously hypertensive rats received monosodium glutamate during the neonatal period. The animals were allocated to the following groups: MS (sedentary metabolic syndrome), MS-T (trained on a treadmill for 1 hour/day, 5 days/week for 10 weeks), H (sedentary spontaneously hypertensive rats) and H-T (trained spontaneously hypertensive rats). The Lee index, blood pressure (tail-cuff system), insulin sensitivity (insulin tolerance test) and functional capacity were evaluated before and after 10 weeks of training. Glucose transporter Type 4 expression was analyzed using Western blotting. The data were compared using analysis of variance (ANOVA) (p<0.05).
RESULTSAt baseline, the MS rats exhibited lower insulin sensitivity and increased Lee index compared with the H rats. Training decreased the body weight and Lee index of the MS rats (MS-T vs. MS), but not of the H rats (H-T vs. H). There were no differences in food intake between the groups. At the end of the experiments, the systolic blood pressure was lower in the two trained groups than in their sedentary controls. Whole-body insulin sensitivity increased in the trained groups. Glucose transporter Type 4 content increased in the heart, white adipose tissue and gastrocnemius muscle of the trained groups relative to their respective untrained groups.
CONCLUSIONIn conclusion, the present study shows that an isolated aerobic exercise training intervention is an efficient means of improving several components of metabolic syndrome, that is, training reduces obesity and hypertension and increases insulin sensitivity.
Metabolic syndrome is associated with an increased risk of developing diabetes (1) and cardiovascular mortality (2). The link between insulin resistance, inflammation and obesity (3) is the most widely accepted hypothesis for the development of metabolic syndrome, and treatments for this syndrome should primarily address each of these elements. GLUT4 is a protein involved in the facilitated diffusion of glucose in insulin-sensitive tissues, and it plays a major role in tissue and plasma glucose balance. In unstimulated cells, GLUT4 is stored intracellularly in specialized compartments known as GLUT4 storage vesicles (GSVs), which participate in the cycling of GLUT4 to and from the plasma membrane through slow exocytosis from GSVs and fast endocytosis from the cell surface (4–6). Changes in the expression and/or translocation of this glucose transporter, especially in adipose and skeletal muscle cells, has been directly associated with insulin resistance (7). In metabolic syndrome, obesity, hypertension and diabetes are associated with insulin resistance and therefore, with reduced GLUT4 expression (8).
Aerobic exercise training is an effective intervention for improving insulin sensitivity because it increases glucose transport in insulin-sensitive tissues, especially skeletal muscle (9). This benefit results from increases in both GLUT4 gene expression (10) and the translocation of vesicles containing GLUT4 from the cytoplasm to the cell surface (11,12), among other factors (4,13).
Similar benefits have been described in patients with metabolic syndrome who showed improved insulin sensitivity and pancreatic β-cell function after interval training (14). In addition, when combined with a low-calorie diet and salt restriction, aerobic exercise training can reduce both blood pressure and the prevalence of metabolic syndrome (15). Therefore, the most appropriate approach for treating metabolic syndrome includes lifestyle changes, such as aerobic exercise training, and healthy eating habits. However, few studies have investigated either the isolated effects of aerobic exercise training in humans with metabolic syndrome or the benefits this training provides for most risk factors (16).
In our laboratory, the administration of MSG in spontaneously hypertensive rats (SHR) has been shown to provide a suitable animal model for studying metabolic syndrome by providing a phenotype similar to that seen in humans. This phenotype includes obesity, insulin resistance, dyslipidemia, inflammation and the maintenance of hypertension (17). Furthermore, reduced GLUT4 content in the insulin-sensitive tissues of MSG animals has been reported as an insulin resistance-related mechanism (18,19).
Because there are no data in the literature showing the effects of aerobic exercise training without dietary changes in an animal model of metabolic syndrome, the present study aimed to evaluate the potential benefits of aerobic exercise training on insulin sensitivity, GLUT4 protein expression and blood pressure in rats with metabolic syndrome.
MATERIALS AND METHODSAll animal experimentation procedures followed the Canadian Council on Animal Care guidelines. The study was approved by the Research Ethics Committee of the Instituto de Cardiologia do Rio Grande do Sul, Protocol #UP:4330. All of the animals were bred and kept under standard laboratory animal housing conditions at the Animal Production and Research Unit of the Center for Scientific and Technological Development of State Foundation for Production and Research in Health of Rio Grande do Sul, Brazil. The animals were fed a standard, balanced rat chow, provided with water ad libitum and kept in special cages exposed to a 12-hour light and 12-hour dark cycle (6 a.m./6 p.m.) in a 20 to 25°C temperature-controlled room.
We studied 20 male spontaneously hypertensive rats, 10 of which received a subcutaneous injection of MSG (5 mg/kg of body weight) diluted in 0.9% saline during their first 10 days of life (MS group). The remaining animals (H group) received saline injections during the same period. The Lee index (cube root of the body weight divided by the nasoanal length) was also evaluated (20). Seven months after weaning, the rats were divided in four subgroups (n = 5 per group): sedentary (MS and H) and trained (MS-T and H-T).
Food intake was monitored weekly. Once a week, the food was weighed before it was given to the animals and again at the same time the next day, after it was removed from the cages. This procedure allowed us to calculate the relative food intake (chow [g]/body weight [g]) of each animal during the 10 weeks of the protocol. The area under the curve (AUC) was calculated using the relative food intake.
Maximal exercise testFunctional capacity was measured using the maximal exercise test, as described in the literature (21). First, all of the rats were subjected to an adaptation period on the treadmill (Inbramed, Brazil) at a speed of 0.3 km/h for 15 minutes for three consecutive days. The maximal exercise test was then performed individually at an initial speed of 0.3 km/h with increments of 0.3 km/h every three minutes. The maximal exercise test was performed before and after the exercise training period. An intermediate test was performed five weeks after initiating the training program to make any adjustments to the exercise intensity that were necessary to account for the expected adaptations.
Exercise trainingDynamic aerobic exercise training, prescribed based on the maximal exercise test, was performed at low-moderate intensity (∼50% to 70% of the maximal running speed during the maximal exercise test) for one hour a day, from 7 to 8 p.m., five days a week for 10 weeks, with a gradual increase in speed from 0.6 to 1.2 km/hr.
Blood pressureBlood pressure was measured using a tail-cuff system (Model 229, IITC Life Science Inc, Woodland Hills, California, USA) before and at the end of the exercise training period (24 h after the last bout of exercise). All of the animals were placed in a restrainer for 15 minutes, a cuff was attached to their tail, and the blood pressure was recorded. For both measures, the rats underwent an adaptation period of seven days to familiarize them with the procedure, which began right after the adaptation period. Valid blood pressure values were obtained as described at the 8th day of measurements. The blood pressure was measured before and after the exercise training period.
Insulin tolerance testAn insulin tolerance test was performed in the manner described in the literature (22) using commercially available regular insulin (Humulin, Eli Lilly, São Paulo, Brazil). The insulin tolerance test for the trained groups (MS-T and H-T) was performed 24 hours after the last exercise bout to avoid exercise-induced acute effects (23) and at the same time of the day for their respective control groups (MS and H). After three hours of food deprivation, the rats were anesthetized (ketamine: 160 mg/kg body weight and xylazine: 10 mg/kg body weight), and insulin (0.75 U/kg) was administered into the vein of the penis. This test involves measuring blood glucose using test strips (Accu-chek Advantage, Roche, Mannheim, Germany) six times: at baseline (before insulin administration) and 4, 8, 12, 16 and 20 minutes post-insulin application. The measured glucose values were transformed by taking the natural logarithm of these values: the slope of linear regression between the transformed data and time was calculated and multiplied by 100 to obtain the glucose decay constant rate (kITT) as a percentage per minute (%.min-1). The insulin tolerance test was performed before and after the exercise training period.
Tissue preparation and Western blotTissue samples were removed from the sedentary and trained animals 40 hours after the last exercise bout to avoid measuring the last exercise session's acute effects on the GLUT4 expression and insulin sensitivity (24). For this procedure, the rats were fed normally and then anesthetized with a high dose of pentobarbital sodium (100 mg/kg). The entire gastrocnemius muscle was removed from the right hind limb without separating red or white fibers for use in the skeletal muscle homogenate. After the removal of the white fat tissue (epididymal tissue), a thoracotomy was performed, and the entire heart was immediately removed before respiratory arrest occurred. The cardiac muscle homogenate was obtained from the whole heart.
All of the tissue samples were homogenized and processed to obtain a subcellular fraction enriched with plasma membrane proteins for further analysis of GLUT4, as previously described by Machado et al. (25). Briefly, the fat tissue was homogenized using a Polytron homogenizer (Marconi, Piracicaba, Brazil) at 20,000 rpm for 30 seconds in buffer (7.4 pH, 10 mmoL/L Tris-HCl, 1 mmoL/L EDTA and 250 mmol/L sucrose) at a weight:volume ratio of 1:4 and centrifuged at 2,000 g for 15 minutes at 4°C. The supernatant containing the free-fat extract (FFE) was separated and centrifuged at 12,000 g for 15 minutes at 4°C. The pellet, which corresponded to the plasma membrane fraction, was resuspended in 1 mL of buffer. The gastrocnemius and heart were homogenized in the same buffer and centrifuged at 1,000 g for 10 minutes. The supernatant was separated, and the pellet was resuspended into 1/3 of the initial volume and centrifuged again at 1,000 g for 10 minutes. Both supernatants were mixed and centrifuged at 147,000 g for 75 minutes. The final pellet was resuspended in 1 mL of buffer, corresponding to the plasma membrane fraction.
The GLUT4 concentration in the plasma membrane (PM) fraction was determined using a Western blot. Hundred microgram specimens were solubilized in NuPAGE buffer (Invitrogen, Carlsbad, USA), subjected to SDS/PAGE (10%) and transferred to a nitrocellulose membrane. The membranes were incubated with anti-GLUT4 antibody (#07-1404, Millipore, Billerica, USA), which was titrated to 1:1,000, overnight at 4°C. The membranes then underwent an additional three-hour incubation at 37°C and were washed (0.05% PBS/Tween 20, 1×15 min, 3×5 min) and incubated with a secondary antibody, 1:10,000 goat anti-rabbit IgG (#12-349, Millipore, Billerica, USA). Afterward, the membranes were rewashed in PBS/Tween 20, incubated in the dark with peroxidase substrate (ECL kit, GE Healthcare, New York, USA) and exposed to an ultrasensitive radiographic film (Kodak, Frankfurt, Germany). The blot intensity was quantified using optic densitometry with public domain software (Scion Image). For loading control, densitometric analysis of the total protein in the lanes was performed between 35 and 130 kDa (based on the Page Ruler Prestained Protein Ladder®, Thermo Scientific, USA) in Ponceau-stained membranes. These values were used to normalize the respective GLUT4 values (26). The final results were expressed in arbitrary units (AU).
Statistical analysisThe results were compared using two-way or repeated measures ANOVA, as applicable, and Bonferroni's post-hoc test and were described as means and standard deviations. Pearson's correlation was used to study the association between the variables. SPSS statistical software for Windows was used for the ANOVA and Pearson's correlation analyses. The area under the curve of the intake rate relative to body weight was calculated using GraphPad Prism Software. A 5% level of significance was set for all of the tests.
RESULTSTable 1) shows the body weight, Lee index, insulin sensitivity and maximum speed achieved during the maximal exercise test before and after the aerobic exercise training period. The comparisons shown in Table 1) refer to evaluations that occurred at the same time. As expected, at baseline, the MS rats had lower body weights and insulin sensitivity but increased Lee index compared with the H rats (p = 0.016, p = 0.001 and p<0.001, respectively). The functional capacity, which was evaluated using the maximal exercise test, was similar for the MS and H groups.
Effect of exercise training on the studied animals.
| Initial | Final | Initial | Final | |||
|---|---|---|---|---|---|---|
| Body weight (g) | H | 336±17 | 345±12 | H-T | 332±20 | 358±14 |
| MS | 289±30∗ | 301±25∗ | MS-T | 273±41† | 246±49†‡ | |
| Lee index | H | 0.28±0.01 | 0.28±0.01 | H-T | 0.28±0.01 | 0.29±0.01 |
| MS | 0.33±0.1∗ | 0.36±0.01∗ | MS-T | 0.33±0.02† | 0.29±0.01‡ | |
| kITT (%.min-1) | H | 3.9±0.5 | 3.8±0.5 | H-T | 3.9±0.4 | 4.7±0.3‡ |
| MS | 2.8±0.4∗ | 2.9±0.9∗ | MS-T | 3.0±0.5† | 4.3±0.6‡ | |
| Maximal exercise test | H | 1.4±0.3 | 1.2±0.2 | H-T | 1.4±0.2 | 2.3±0.3‡ |
| (km/h) | MS | 1.3±0.3 | 1.0±0.2 | MS-T | 1.4±0.2 | 2.4±0.1‡ |
Aerobic exercise training did not change the body weight or Lee index, but it increased the insulin sensitivity in hypertensive rats (H-T) by ∼21% (p = 0.041) relative to that of their controls (H group). In MS-T rats, the aerobic exercise training reduced the body weight (∼10%, p = 0.033) to below that of their sedentary controls (MS group). Similarly, there was a decrease in the Lee index (∼12%, p<0.001) in the MS-T rats compared with their sedentary controls (MS group) in response to the 10-week aerobic exercise training. Insulin sensitivity increased by ∼43% (p = 0.012) in the MS-T group over that of the MS group and was similar to that of the H group (Table 1).
There were no differences in food intake between the groups throughout the experimental period, although there was a trend towards less food consumption by the MS and MS-T rats compared with the H and H-T rats (p = 0.053) (Figure 1).
Smooth curve of relative food intake (chow [g]/body weight [g]). H (n = 5): sedentary spontaneously hypertensive rats; H-T (n = 5): trained spontaneously hypertensive rats; MS (n = 5): sedentary metabolic syndrome rats; MS-T (n = 5): trained metabolic syndrome rats. The detailed view shows the area under the curve (AUC). One-way ANOVA was used to compare the AUCs. There were no differences in food intake between the groups (p>0.05).
The functional capacity increased by ∼100% in the H-T group compared with the H group (p<0.001) and by ∼125% in the MS-T group compared with the MS group (p<0.001), showing that the H-T and MS-T rats were actually well-trained at the end of the study period.
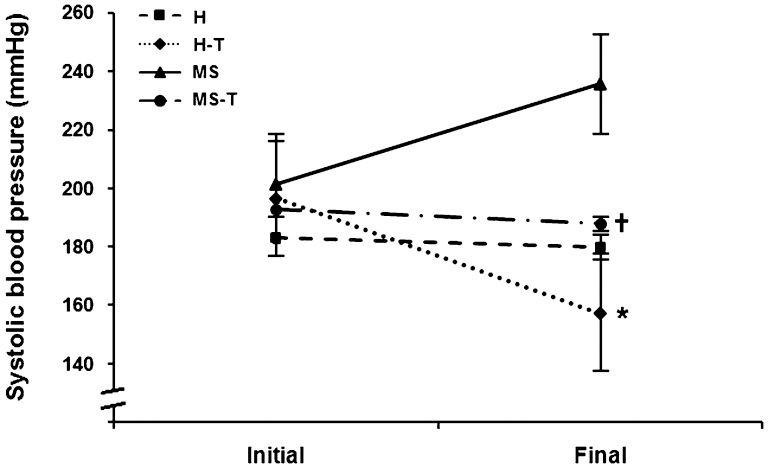
Figure 2) shows the 10-week training program's effect on systolic blood pressure. At the end of the experiments, both of the trained groups showed lower systolic blood pressure compared with their sedentary controls (H-T vs. H, p = 0.010 and MS-T vs. MS, p<0.001). A comparison of the systolic blood pressure after the training period to the baseline levels revealed a reduction in blood pressure in the rats from the H-T group and no change in the rats from the M-T group.
Effect of 10-week aerobic exercise training on systolic blood pressure. H (n = 5): sedentary spontaneously hypertensive rats; H-T (n = 5): trained spontaneously hypertensive rats; MS (n = 5): sedentary metabolic syndrome rats; MS-T (n = 5): trained metabolic syndrome rats. Repeated measures ANOVA: p(group)<0.001; p(time) = 0.344; p(interaction)<0.001. Bonferroni's post-hoc test group: ∗ p = 0.010 vs. H; † p<0.001 vs. MS.
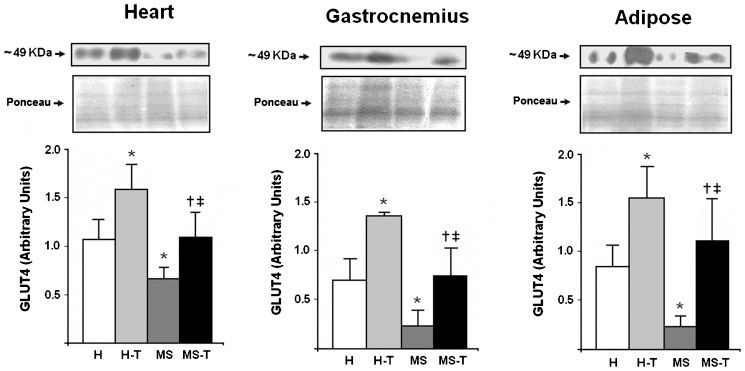
Figure 3) shows the GLUT4 content in the heart (A), gastrocnemius muscle (B) and white adipose tissue (C) in the studied groups. MS rats showed lower GLUT4 expression in all examined tissues (heart, p = 0.036; gastrocnemius muscle, p = 0.007; and adipose tissue, p = 0.022) as compared with H rats. In the H-T group, aerobic exercise training effectively increased the GLUT4 content in the plasma membrane by ∼48% in the heart (p = 0.041), ∼98% in the gastrocnemius muscle (p<0.001) and ∼86% in the adipose tissue (p = 0.028) compared with the H group. Similar to the difference observed in the H-T group vs. the H group, though more remarkable, the MS-T animals also showed increased GLUT4 levels in the heart (∼67%, p = 0.001), gastrocnemius muscle (∼237%, p<0.001) and white adipose tissue (∼386%, p<0.001) in response to the 10-week aerobic training compared with the MS group (Figure 3). Despite the improvements induced by aerobic exercise training in all of the tissues from the MS-T animals, their GLUT4 content was not as high as that of the trained hypertensive rats (H-T) but was similar to that of the H group (Figure 3).
GLUT4 protein content in the heart, gastrocnemius muscle and epididymal white adipose tissue. The top shows representative images of GLUT4 and the respective loading controls. The loading controls are the total proteins in the 35- to 130-kDa range of Ponceau-stained membranes, as described in the Methods section. The graphs on the bottom show the means±SEM. H (n = 5): sedentary spontaneously hypertensive rats; H-T (n = 5): trained spontaneously hypertensive rats; MS (n = 5): sedentary metabolic syndrome rats; MS-T (n = 5): trained metabolic syndrome rats. Two-way ANOVA (Bonferroni's post-hoc test). ∗ p<0.05 vs. H; † p<0.05 vs. MS; ‡ p<0.05 vs. H-T.
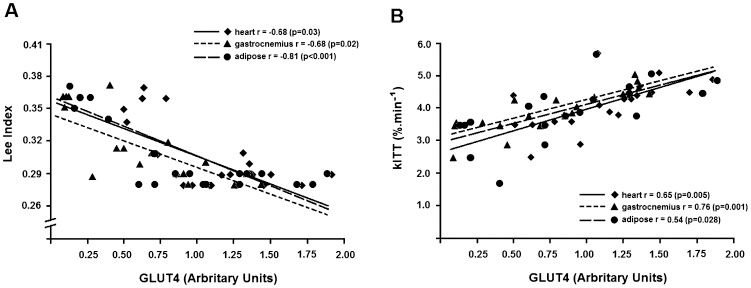
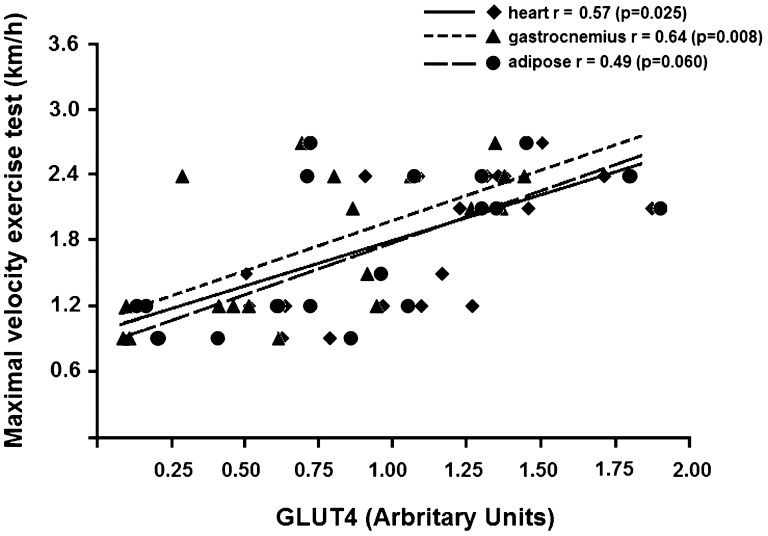
Figure 4A) shows the correlations of the GLUT4 content in the heart, gastrocnemius muscle and adipose tissue with the Lee index of all of the studied animals. An inverse association was found between the GLUT4 protein content and the Lee index in all insulin-sensitive tissues. The highest correlation was seen in the adipose tissue. Figure 4B) shows a positive correlation between the GLUT4 content in insulin-sensitive tissues and the insulin sensitivity, as evaluated using kITT. Of the studied tissues, the gastrocnemius muscle showed the highest correlation between the GLUT4 protein content and insulin sensitivity. Figure 5) shows a moderate correlation between the GLUT4 protein content in the heart and the gastrocnemius muscle and the maximal speed obtained during the exercise test. No correlation was observed between these variables for the adipose tissue.
Our study shows the results of an intervention using aerobic exercise training in an animal model of metabolic syndrome. At the beginning of the study, the studied animal model (MSG-treated SHR) showed similar characteristics to those seen in metabolic syndrome in humans, such as hypertension, obesity and insulin resistance. Reduced GLUT4 contents in the heart, skeletal muscle and adipose tissue were associated with these abnormalities. Aerobic exercise training, even without dietary restrictions, was beneficial: the insulin sensitivity improved, the Lee index decreased, the GLUT4 expression in insulin-sensitive tissues increased, and high blood pressure levels were avoided.
Spontaneously hypertensive rats treated with MSG developed obesity and insulin resistance, which was confirmed by the ∼70% reduction in the values obtained for the insulin tolerance test compared with those obtained for rats that were not treated with MSG. Furthermore, SHRs present spontaneous development of systemic hypertension (27), which is not observed in MSG-only treated animals and corroborates the appropriateness of the proposed animal model for studying metabolic syndrome as manifested in humans. The body weight of the MS rats was lower than that of the H rats. However, the MS rats were, in fact, obese, which is evinced by their high scores on the Lee index, a parameter that has been demonstrated to be appropriate for assessing obesity rodents (28,29). Moreover, the rats' food intake relative to body weight was similar for the two groups, showing that the aerobic exercise training did not affect the rats' appetite and that the MSG-treated animals did not develop hyperphagia. Indeed, these data clearly show that the MSG-treated rats (MS and MS-T) had lower energy expenditures because their food intake was similar to that of their controls (H and H-T); however, their overall fat mass was greateer, resulting in short (nasoanal length) but proportionally obese animals (30) with insulin resistance (31). Furthermore, as our group recently described (17), a significant reduction in GLUT4 expression was seen in all tissues from MS rats compared with those from H animals, especially the gastrocnemius muscle and adipose tissues.
In this study, we have shown that aerobic exercise training has a beneficial effect on some of the characteristics of metabolic syndrome. It also increases the trained animals' aerobic capacity and reduces the Lee index, which is associated with lower body weight. Exercise training promotes higher metabolic rates, which can contribute to decreased fat content and body weight, as observed in obese rats (32). This increase in metabolic rates was also confirmed in the present study because despite an unaltered food intake, the MS rats had reduced body weights and lower Lee index scores. These aerobic exercise training effects were accompanied by improved insulin resistance, which was previously observed in MSG-obese mice that had partial compensation of the hyperinsulinemic state (33), and in high-fat-diet-fed rats whom recovered insulin sensitivity in skeletal muscle after exercise training (34).
Interestingly, the aerobic exercise training was only observed to lower blood pressure in the hypertensive rats that did not receive MSG. However, the MS-T rats also benefitted from the aerobic exercise training because their blood pressure levels did not increase, whereas those of the sedentary control group (MS) did. In an earlier study, our research group compared the blood pressure behaviors of Wistar-Kyoto (WKY) rats, SHR and rats with metabolic syndrome (SHR injected with MSG) during metabolic syndrome development until the rats were nine months old. Both the hypertensive-only rats and the rats with metabolic syndrome had higher blood pressure levels than did the normotensive rats at all of the studied ages (17). Furthermore, as the present study shows, aerobic training did not induce any change in the blood pressure of the WKY rats, whereas the blood pressure was reduced by 9% in the hypertensive rats (35). Consistent with these findings, voluntary physical exercise was shown to cause vasodilation through decreased oxidative stress (36) and increased nitric oxide (NO) bioavailability (37). In patients with metabolic syndrome who underwent high-intensity intermittent exercise or moderate-intensity continuous exercise, the body mass index, waist circumference and blood pressure were reduced, while the HDL cholesterol levels and insulin sensitivity increased. Moreover, at the end of the study, 46% of the subjects in the high-intensity training group were no longer diagnosed with metabolic syndrome (14).
The mechanisms by which dietary changes and aerobic exercise training promote improvements in metabolic syndrome patients have some common aspects because both lead to reductions in weight, visceral fat and inflammatory markers. In caloric restriction, these changes occur due to reduced fat intake associated with a concomitant increase in the consumption of antioxidant food, that can per se reduce blood pressure and oxidative stress induced by hyperlipidemia and hyperglycemia, as well as improved insulin sensitivity, since the inflammatory state seems to be the mechanism by which obesity and insulin resistance are linked (38). Exercise training contributes to increases in the metabolic rate by improving cardiovascular capacity and suppressing pro-inflammatory cytokine production, in addition to increasing the release of nitric oxide and the consumption of free fatty acids, thus enhancing insulin sensitivity (14).
By applying aerobic exercise training to animals with metabolic syndrome, we obtained new results concerning the GLUT4 content of the plasma membranes of the heart, skeletal muscle and adipose tissue, which increased by 67%, 237% and 386%, respectively. Although these results are impressive, the absolute GLUT4 content in the tissues from the MS-T animals was still lower than that observed in the H-T animals because the GLUT4 content was lower in the MS rats prior to the aerobic exercise training. Several mechanisms may explain the difference between the GLUT4 expression in the MS and H animals. In obesity, the inflammatory state is the major cause of insulin resistance. MSG-treated mice present macrophage infiltration in adipose tissue accompanied by increased release of inflammatory cytokines, such as TNF-α and IL-6, which stimulate the NF-κB-mediated inflammatory pathway and repress GLUT4 protein expression (19). This GLUT4 repression most likely occurs in rats with metabolic syndrome. A direct comparison of the effects of dietary modification and aerobic exercise training was reported by Ferrier et al. (2004) in obese elderly subjects. Both interventions led to improved insulin sensitivity. However, this improvement was caused by a reduction in TNF-α with no change in skeletal GLUT4 in the dietary modification group and by an increase in skeletal GLUT4 with no change in TNF-α in the exercise intervention group. Applying these data to our own results might indicate that the remission of the insulin-resistant state was incomplete in the MS rats because of persistent inflammation that was not offset by the proposed intervention (39).
We found an inverse association between the GLUT4 expression and the Lee index similar to that observed in other studies in which the GLUT4 content in MSG-mice increased after low-calorie-diet-induced weight loss (18). This association was remarkable in adipose tissue, in accordance with this tissue's central role in the development of obesity. There was also a positive correlation between GLUT4 expression and insulin sensitivity, which increased in the studied tissues, most notably in the gastrocnemius muscle. The relationship between GLUT4 and insulin sensitivity has been extensively reported, and transgenic animals with a knockout or overexpression of muscle GLUT4 are able to evince its key role in insulin sensitivity (40).
Our study showed a moderate correlation between the gastrocnemius GLUT4 content and the maximal exercise test; this correlation was not shown for GLUT4 in the heart or adipose tissue. Aerobic exercise training increases the oxidative capacity of the exercised muscle (41), which is consistent with its higher energy production and increased ability to perform maximal exercise.
Some limitations of the present study should be noted. We did not study specific groups for a low-calorie diet intervention only, exercise training only or both interventions together. Thus, we could not compare the magnitude of the benefits of each intervention (low-calorie diet or exercise training) for animals with metabolic syndrome. However, our research has clinical implications; this study provides data that support the use of exercise training as a treatment regardless of dietary changes because exercise training represents an efficient nonmedical intervention for most of the components of metabolic syndrome.
In conclusion, in the presented animal model, aerobic exercise training as an isolated intervention for treating metabolic syndrome was demonstrated to be efficacious for improving several metabolic afflictions. The proposed treatment enhanced insulin sensitivity and increased the GLUT4 expression in insulin-sensitive tissues. The treatment was able to reduce the Lee index score and the body weight and prevent an increase in systolic blood pressure in rats with metabolic syndrome.
AUTHOR CONTRIBUTIONSCaponi PW was involved in the conception and design of the study, collection, analysis and interpretation of the data, and drafting and editing of the final version of the manuscript for publication. Lehnen AM participated in data collection (maximal exercise test, blood pressure and molecular analysis), statistical analysis and editing of the final version of the manuscript for publication. Pinto GH and Borges J were equally involved in the data collection (exercise training, tissue and blood collection and molecular analysis). Markoski M was involved in the collection (sample preparation and molecular analysis), analysis and interpretation of the data. Machado UF was involved in the conception and design of the study, data analysis and interpretation and the review of all parts of the final version of the manuscript for publication. Schaan BD was involved in the conception and design of the study, analysis and interpretation of the data, and drafting, writing and editing of the final version of the manuscript for publication. All of the authors read and approved the final version of the manuscript.
No potential conflict of interest was reported.






![Smooth curve of relative food intake (chow [g]/body weight [g]). H (n = 5): sedentary spontaneously hypertensive rats; H-T (n = 5): trained spontaneously hypertensive rats; MS (n = 5): sedentary metabolic syndrome rats; MS-T (n = 5): trained metabolic syndrome rats. The detailed view shows the area under the curve (AUC). One-way ANOVA was used to compare the AUCs. There were no differences in food intake between the groups (p>0.05). Smooth curve of relative food intake (chow [g]/body weight [g]). H (n = 5): sedentary spontaneously hypertensive rats; H-T (n = 5): trained spontaneously hypertensive rats; MS (n = 5): sedentary metabolic syndrome rats; MS-T (n = 5): trained metabolic syndrome rats. The detailed view shows the area under the curve (AUC). One-way ANOVA was used to compare the AUCs. There were no differences in food intake between the groups (p>0.05).](https://static.elsevier.es/multimedia/18075932/0000006800000007/v1_202212011300/S1807593222016751/v1_202212011300/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)



