Our study aimed to investigate the impact of fatigue on the severity of stroke and to explore the underlying mechanisms.
METHODS:Fatigued male rats underwent middle cerebral artery occlusion and the infarcted brain area was determined. Then, coagulation parameters were assessed in the fatigued group and a control group. In addition, the level of fibrinogen was determined in rats deprived of sleep for various numbers of days. To study whether interleukin-6 was involved in fibrinogen synthesis during fatigue, we also measured levels of interleukin-6 in rats deprived of sleep for various numbers of days. Furthermore, brain injury by middle cerebral artery occlusion was measured in wild-type mice, interleukin-6-/- mice and wild-type mice treated with bezafibrate.
RESULTS:More severe cerebral infarction was observed in the fatigued rats, resulting in an infarct ratio of 23.4%. The infarct ratio was significantly increased in the fatigued rats compared with that in the control group (8%, p<0.05). The level of fibrinogen was increased significantly in the fatigued rats compared with that in the control group. In addition, a marked reduction in fibrinogen level was observed in the fatigued interleukin-6-/- mice compared to their wild-type counterparts, whereas no difference was observed between fatigued wild-type mice and interleukin-6-/- rats treated with recombinant human interleukin-6. The reduction in brain injury due to middle cerebral artery occlusion during fatigue was observed in interleukin-6-/- mice and wild-type mice treated with bezafibrate.
CONCLUSION:Fatigue could increase stroke severity and was associated with the interleukin-6-induced expression of fibrinogen.
Fatigue has been found to be prevalent in the general population, and it is an important symptom of mental and stress-related health complaints (1-3). The high prevalence of this condition in the general population has led to a growing interest in the influence of clinical and sociodemographic factors on the level of fatigue experienced by individuals (4,5). In previous studies, fatigue was shown to be strongly associated with bad mental health states, impaired functioning and a variety of long-term illnesses; it is also a central symptom in many diseases, such as ischemic heart disease, cancer and depression (6-10). It has been reported that fatigue is the most common prodromal symptom before acute myocardial infarction, and preinfarction fatigue could contribute to early left ventricular dysfunction (11,12). In addition, fatigue is also a common symptom following a stroke (13,14). Numerous studies have confirmed that severe fatigue after stroke usually predicts a bad prognosis and an increased risk of recurrent stroke (15,16). However, few studies have examined the influence of fatigue on the severity of acute ischemic events, such as acute ischemic stroke. Therefore, our study aimed to investigate the impact of fatigue on the severity of stroke and to explore the underlying mechanisms.
MATERIALS AND METHODSAnimalsRats and mice were housed in a controlled environment and provided access to standard rodent chow and water. The animal care practices were in compliance with Chinese regulations on the protection of animals used for experimental and other scientific purposes.
Experimental groupsTo study the effects of fatigue on the cerebral infarction area, two groups of Sprague-Dawley rats were assigned randomly to control and fatigued groups. To study whether IL-6 was involved in the synthesis of fibrinogen during fatigue, rats and mice were randomly assigned to four groups and six groups, respectively. Each group of rats was deprived of sleep for a different length of time: no sleep deprivation (control), sleep deprivation for one day (F1), sleep deprivation for three days (F3) and sleep deprivation for five days (F5). The six groups of mice included wild-type mice, fatigued wild-type mice, IL-6-/- mice, fatigued IL-6-/- mice, IL-6-/- mice treated with IL-6 and fatigued IL-6-/- mice treated with IL-6. To study whether the decreased expression of fibrinogen could affect the infarction area, three groups of mice were tested: a fatigued group, a fatigued IL-6-/- group and a fatigued group treated with bezafibrate.
All of the studies included six rats or mice per group.
Fatigue modelMale Sprague-Dawley rats were deprived of rest for five consecutive days in a cage filled with water to a height of 1.5 cm, as described previously (18). The cage was 485x350x200 mm, and there was a heater placed underneath it to keep the water warm. Under these conditions, the rats were unable to assume a resting position while avoiding the water, and they progressively grew fatigued.
MCAO model and evaluation of infarcted areaAfter fatigue treatment (five days of sleep deprivation), male Sprague-Dawley rats underwent the MCAO procedure described by Longa et al. (17). Briefly, the rats were anesthetized with ketamine and xylazine, and the left common carotid artery was exposed. Then, the external carotid artery and its branches were isolated and coagulated. A 3-0 Nylon suture with a blunted tip was inserted into the internal carotid artery through the external carotid artery stump and then advanced to the anterior cerebral artery to occlude the middle cerebral artery (MCA). The skin was then sutured, and the rats were allowed to awaken. Twenty-four hours after the operations, the rats were sacrificed, and coronal sections of the brain (2 mm thick) were cut and immersed in a 2% solution of 2,3,7-triphenyltetrazolium chloride. The stained slices were then fixed by immersion in phosphate-buffered 4% paraformaldehyde. The infarcted area and hemispheric area of each section were traced and measured using an image-analysis system (a Macintosh computer running the public domain National Institutes of Health Image program, written by Wayne Rasband and available on the Internet). The percentage of infarction (infarct ratio) was calculated by dividing the infarcted area by the total area of the ipsilateral hemisphere.
Measurement of coagulation parametersBlood samples were obtained from the rats via a nonheparinized venous catheter in the femoral vein. APTT, PT and TT (Taiyang Biotechnology Company, Shanghai, China) were measured automatically from clotting tests using commercial reagents, and the test results were reported in seconds. The default settings for the minimal/maximal measuring times for APTT and TT were 3/120 sec and 13/240 sec, respectively. Fibrinogen was detected using Claus's method with a human plasma calibration standard provided by the manufacturer.
Measurements of IL-6 in serum and CSFThe levels of IL-6 in the serum/CSF were measured using commercially available ELISA kits (IL-6, Fuji Lebio, Tokyo, Japan). The kit had a sensitivity limit of 4 pg/ml, and no detectable IL-6 was found in sera from control mice or IL-6 knockout mice.
Animal treatmentMale IL-6-/- mice, weighing 18-22 g, were kept on a 12-h day/night rhythm with free access to water and standard rodent chow. The animals were injected daily with 0.02 μg of rhIL-6/g body weight in a 0.9% NaCl solution containing 0.1% mouse serum albumin (MSA) or with only the 0.9% NaCl/0.1% MSA solution at the start of sleep deprivation, and the treatment lasted five days.
Bezafibrate was suspended in 1% methylcellulose solution and administered at a dose of 10 mg/kg/day orally at the start of sleep deprivation and the treatment lasted five days. The same amount of methylcellulose vehicle solution was also administered orally as a control.
CSF and tissue samplesAfter five days of sleep deprivation, the fatigued rats were anesthetized with 350 mg/kg ip chloral hydrate, and CSF (60-100 μl) was drawn from the cisterna magna using a glass capillary with a tip of approximately 300 μm in size. The surgery was performed carefully to avoid blood contamination. The CSF was then prepared for an IL-6 enzyme-linked immunosorbent assay (ELISA).
The brain samples were placed in sterile PBS containing a protease inhibitor cocktail (0.2 mM 4-[2-aminoethyl]benzenesulfonyl fluoride, HCl [AEBSF], 1 μg/ml aprotinin, 1 mM benzamidine, 1 mM EDTA, 10 μg/ml leupeptin and 10 μg/ml of pepstatin) and then homogenized and centrifuged (10000 g, 30 min, 4°C). Subsequently, the supernatant was removed and stored at -70°C. All of the samples were assayed for immunoreactive IL-6 using a validated rat-specific ELISA kit (IL-6, Fuji Lebio, Tokyo, Japan). Briefly, total brain samples were added to wells and incubated for two hours. Then, the conjugate was added and incubated for another two hours. After washing five times, the substrate solution was added and incubated for 30 minutes. Finally, the stop solution was added and measurements were obtained at 450 nm.
RESULTSEffects of fatigue on the cerebral infarction areaTo study the impact of fatigue on the severity of stroke, we compared the cerebral infarction area between the fatigued and control groups. Figure1A presents representative results of the effects of MCA occlusion on infarction size in both groups: the control group and the fatigued group (rats deprived of sleep for five consecutive days). Coronal sections were obtained by cutting brain slices at distances of 2, 4, 6, 8 and 10 mm from the rostral extremity of the frontal cortex. The white-colored areas represent the infraction regions in these sections. Figure1B shows the percentage of infarction area in rats that underwent MCAO. More severe cerebral infarction was observed in the fatigued rats and the infarct ratio was 23.4%. The infarct ratio was significantly increased in the fatigued rats compared with that in the control group (8%, p<0.05).
The cerebral infarction area after MCAO in rats from the various groups. Figure1A shows representative photographs of coronal sections of rat brains. From left to right are the MCAOs in the control rats and fatigued rats, respectively. The white areas represent the infarct regions. Figure1B shows the infarct ratios in rats from the various groups. The infarct ratio was calculated as the percentage of infarcted tissue per ipsilateral hemisphere. In the control group, the infarct ratio was 8%. In the fatigued group, the infarct ratio was markedly increased (24%) over that of the control group. *, p<0.05.
Figure2A shows the plasma changes in PT, APTT and TT in the fatigued and control groups. None of these parameters showed significant changes in the fatigued rats compared with those of the control group. Figure2B shows the plasma changes in fibrinogen in the fatigued and control groups. The level of fibrinogen increased significantly in the fatigued rats compared with that in the control group.
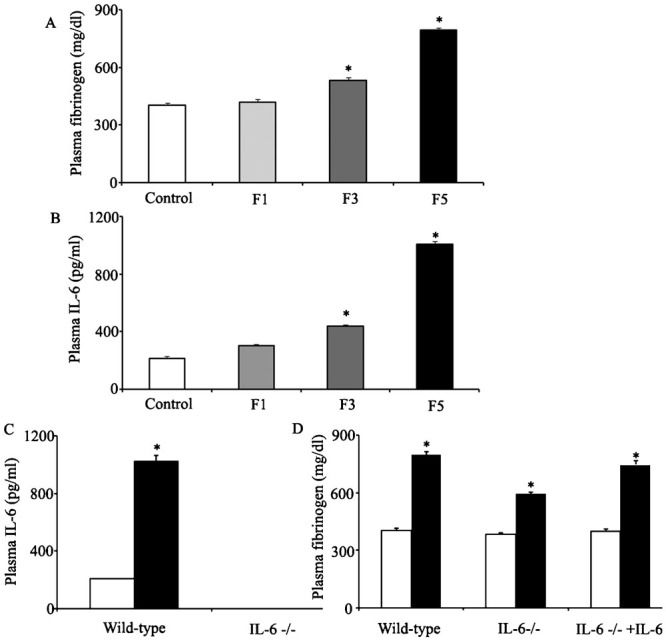
Involvement of IL-6 in fibrinogen synthesis during fatigueAs shown in Figure3A, the plasma fibrinogen level showed slight changes in rats deprived of sleep for one day (F1 group) compared with that in the control group (401.7 mg/dl vs. 417.9 mg/dl). Furthermore, the level was significantly increased in rats deprived of sleep for three days (F3 group); in that group, the fibrinogen level was 532.5 mg/dl. The fibrinogen concentration reached a maximum value in rats deprived of sleep for five consecutive days (F5 group), with a fibrinogen level of 793.1 mg/dl.
Involvement of IL-6 in fibrinogen synthesis. Figures3A and 3B show the changes in plasma fibrinogen and IL-6 levels in rats deprived of sleep for various numbers of days (F1, F3 and F5: deprived of sleep for one day, three days and five days, respectively). Figure3C shows the levels of IL-6 in the plasma of wild-type mice and IL-6-/- mice. Figure3D shows the levels of fibrinogen in the plasma of wild-type mice, IL-6-/- mice and IL-6-/- mice treated with recombinant human IL-6. The white column represents the control group. The black column represents the fatigued group.
To study whether IL-6 was involved in fibrinogen synthesis during fatigue, we also measured the levels of IL-6 in rats deprived of sleep for various time intervals. As shown in Figure3B, the serum IL-6 level started to increase in the F1 group, rose gradually in the F3 group and reached a peak in the F5 group. The levels of serum IL-6 were 301.6 pg/ml, 438.4 pg/ml and 1004.6 pg/ml, respectively (the level of IL-6 in the control mice was 212.6 pg/ml, as shown in Figure3A. As indicated by Figure3C, the levels of IL-6 in serum were significantly increased in fatigued rats, consistent with our previous results, and no detectable IL-6 was observed in the IL-6 knockout mice. To identify whether IL-6 was involved in fibrinogen synthesis, IL-6 knockout mice were included in the subsequent studies. Consistent with the previous results, the level of fibrinogen increased significantly in fatigued wild-type mice. The IL-6-/- mice showed significantly increased fibrinogen levels compared to the wild-type mice, but the fibrinogen levels in the fatigued IL-6-/- mice were not as high as those in the fatigued wild-type mice, suggesting that the elevation of fibrinogen levels during fatigue was at least partially attenuated in the IL-6-/- mice (Figure3D), wild-type: 402 mg/dl and 797.9 mg/dl; IL-6 -/-: 383 mg/dl and 593 mg/dl). At the same time, treating IL-6-/- mice with recombinant human IL-6 (Pharma Technology, Hannover, Lower Saxony, Germany) greatly increased the levels of fibrinogen compared with those in the untreated IL-6-/- mice (fibrinogen: 593 mg/dl and 745.4 mg/dl, respectively).
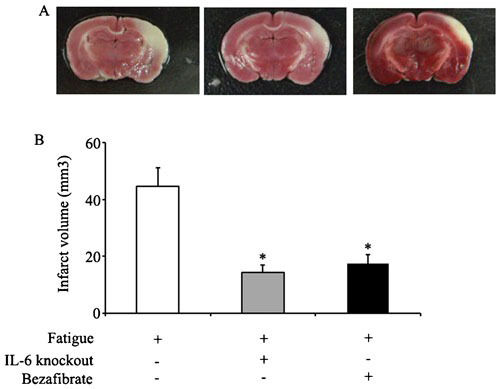
Measurements of cerebral infarction area in fatigued miceThe fatigued mice that underwent sleep deprivation were divided into three groups: 1) fatigued wild-type mice; 2) fatigued IL-6-knockout mice; and 3) fatigued wild-type mice treated with bezafibrate. As shown in Figure4, compared to wild-type animals, the IL-6-/- mice displayed significantly less brain injury after MCAO procedures, demonstrated by the decreased infarction area. At the same time, treating wild-type mice with bezafibrate, which decreased the fibrinogen levels, also provided protection from MCAO-induced brain injury.
Measurements of the cerebral infarction areas. Figure4A shows representative photographs of coronal sections of mouse brains after MCAO. From left to right are the brains of fatigued mice in the control group, in the IL-6-/- group and in the bezafibrate-treated group, respectively. Figure4B shows the infarct volumes in the three groups. *, p<0.05 (compared with the fatigued group).
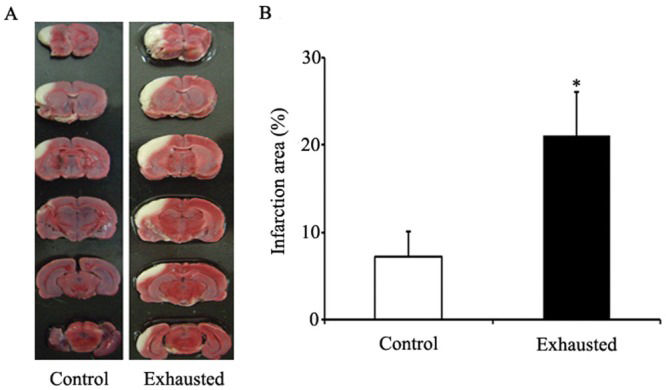
Our results revealed that fatigued rats experienced significantly more brain injury after MCAO compared to their wild-type counterparts. In this study, we used the animal model of fatigue established by Tanaka et al. Although this model was created to study central fatigue, the swimming time of the fatigued rats also decreased sharply. When kept in water, the animals were unable to assume a resting position or sleep soundly as they attempted to avoid the water. Notably, sleep deprivation caused a wide range of neuropsychological and homeostatic changes that could not be ascribed to simple fatigue (19,20). Most of these alterations due to sleep deprivation could have interfered with the cerebral response to ischemic stress. Therefore, we compared brain infarction area in rats performing an acute exercise protocol. In this model of fatigue, the rats were allowed to run until exhaustion, which was defined as the point at which the rats failed to escape the shock grid and had to be repositioned manually to the front of the treadmill on three consecutive occasions (21). This acute exhaustive exercise did not cause neuropsychological or homeostatic changes and could be considered to represent simple fatigue. Subsequent results revealed that the infarction area also increased significantly in rats after performing exhaustive exercise (Supplemental Figure1). These results indicated that fatigue, whether physical or mental, aggregated the brain injury induced by MCAO. Moreover, we explored the underlying mechanisms.
Cerebral infarction area after MCAO in rats of different groups. Supplemental figure 1A shows representative photographs of coronal sections of rat brains. From left to right are the MCAO in the control rats and exhaustive rats respectively. The white areas represent the infarct regions. Supplemental figure 1B shows the infarct ratio in rats of different groups. The infarct ratio was calculated as the % infarcted tissue per ispilateral hemisphere. In the control group, the infarct ratio was 8%. In the fatigue group, the infarct ratio was markedly increased when compared with control group (the ratio was 24%). *, p<0.05.
Fibrinogen is acute-phase protein, and it serves as a nonspecific marker of inflammatory disease (22). It also has important hemostatic properties due to its effects on platelet aggregation and endothelial function. Fibrinogen is a major determinant of plasma viscosity. High levels of fibrinogen in the plasma might reduce blood flow and predispose to thrombosis. High levels of fibrinogen have been associated with an increased risk of cardiovascular disease and stroke (23,24). In our studies, we found that the level of fibrinogen increased significantly in fatigued rats. Whether increased levels of fibrinogen reflected active involvement in the pathogenesis of stroke severity during fatigue or were merely a nonspecific marker of inflammatory disease was not clear. Bezafibrate, a classic lipid-lowering drug, was used to lower plasma fibrinogen concentrations as well (25). In this study, we treated fatigued mice with bezafibrate to lower the fibrinogen levels (data not shown) and then submitted these mice to MCAO surgery. The bezafibrate-treated mice showed significantly decreased brain infarction areas compared to the control mice, indicating that fibrinogen was involved in the pathogenesis of stroke severity.
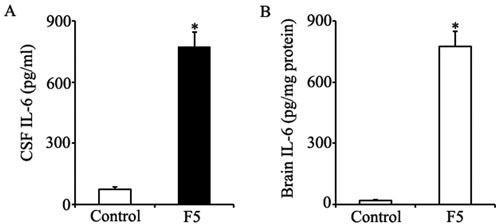
IL-6 is a multifunctional cytokine. Numerous studies have found IL-6-responsive elements in the fibrinogen gene promoter (26). IL-6 and its receptor were involved in fibrinogen synthesis (27). To determine whether elevated fibrinogen levels during fatigue were mediated by IL-6, we first measured the levels of IL-6 in fatigued rats and then examined the fibrinogen concentrations in IL-6-/- mice and wild-type controls. A significant increase in IL-6 levels was observed in the serum of fatigued rats. Because the fatigue model we examined in this study was a mental model, we also measured the levels of IL-6 in the cerebral spinal fluid (CSF) and brain tissue. Fatigue increased the expression of IL-6 in the CSF and brain as well (Supplemental Figures2A and 2B). Consistent with previous results, fatigue increased the expression of fibrinogen in the wild-type controls. A marked reduction in fibrinogen levels was seen in the fatigued IL-6-/- mice compared to their wild-type counterparts, whereas no difference was seen between fatigued wild-type mice and IL-6-/- mice treated with recombinant human IL-6. The reduction in brain injury caused by MCAO during fatigue in IL-6-/- mice suggested that the lack of IL-6 protected against MCAO-induced brain injury. In conclusion, our results explained why fatigued subjects were more prone to severe brain injury induced by MCAO than were control subjects, and IL-6 might serve as a biological marker for fatigue in our fatigue model. To the best of our knowledge, fatigue has typically been considered to be a subjective symptom, and no specific marker has been identified to measure it. People usually underestimate the importance of unusual fatigue, although it might be associated with sudden death due to coronary heart disease or stroke. Our studies demonstrated that progressively increasing levels of IL-6 during fatigue constitute a dangerous signal because they lead to increased expression of fibrinogen, which would eventually aggregate ischemic brain injury. Monitoring the level of IL-6 or the level of fibrinogen during fatigue could help us objectively assess the level of fatigue a person is experiencing. Inhibiting excessive fibrinogen production or reducing the levels of IL-6 might be two effective ways of preventing severe brain injury during fatigue. Because a slight but significant increase in fibrinogen was still observed in fatigued IL-6-/- mice, other pathways might also involve fibrinogen synthesis. There were some limitations of this study that should be acknowledged. First, IL-6 was not the only mediator that induced the expression of fibrinogen during fatigue, and these other mediators should be explored in future studies. Second, the fatigue model (sleep deprivation) applied here was a mental model, whereas combined fatigue (both mental and physical) is more prevalent among people in everyday life; we should therefore develop a more representative fatigue model to study the relationship between the severity of combined fatigue and the volume of the infarcted cerebral area.
Level of IL-6 in CSF and brain tissue. Supplemental figure 2A and 2B show the changes of IL-6 in CSF and brain of rats from different groups. The white column represents the control group. The black column represents the fatigue group (F5: deprived of sleep for five days). The level of IL-6 increased significantly in CSF of fatigue rats when compared with that in control group (771.6±74.2 pg/ml vs. 72.6±10.6 pg/ml). The level of IL-6 increased significantly in brain of fatigue rats when compared with that in control group (775.2±73.6 pg/mg protein vs. 18.3±3.5 pg/mg protein).
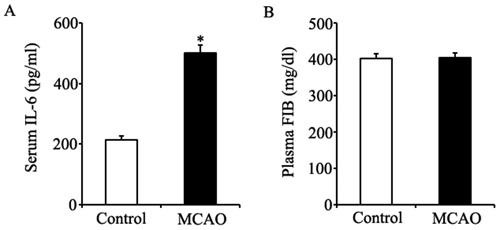
Effect of MCAO on levels of IL-6 and FIB. Supplemental figure 3A shows the serum IL-6 level significantly increased in rats undergoing MCAO operation when compared with that in normal control rats (501.6±25.2 pg/ml vs.212.6±12.9 pg/ml). Supplemental figure 3B shows that the plasma FIB level was not changed in rats undergoing MCAO procedures when compared with that in normal control rats.
This work was supported by grants from the National Postdoctoral Science Foundation of China (20100471802).
AUTHOR CONTRIBUTIONSLei H designed the experiments, detected the levels of fibrinogen and wrote the manuscript. Xu J drew the cerebral spinal fluid and measured the levels of IL-6. Cheng LJ performed the middle cerebral artery occlusion operation. Guo Q and Deng AM supervised the project. Li YS revised the manuscript.
No potential conflict of interest was reported.