To describe the clinicopathological characteristics of patients with upper urinary tract transitional cell carcinomas who are treated surgically and to analyze the occurrence of bladder tumors as well as the development of metastases outside the urinary tract.
MATERIALS AND METHODSThe study comprised a retrospective analysis of 25 patients treated between February 1994 and August 2006. The variables analyzed were: patient age, gender, and clinical presentation; diagnostic methods; pathologic characteristics at the primary site of the tumor (pelvis or ureter); tumor stage and grade; and presence of carcinoma in situ, microvascular invasion and squamous differentiation. The Kaplan-Meier method and the Log-Rank test were used for statistical analysis of bladder recurrence-free survival.
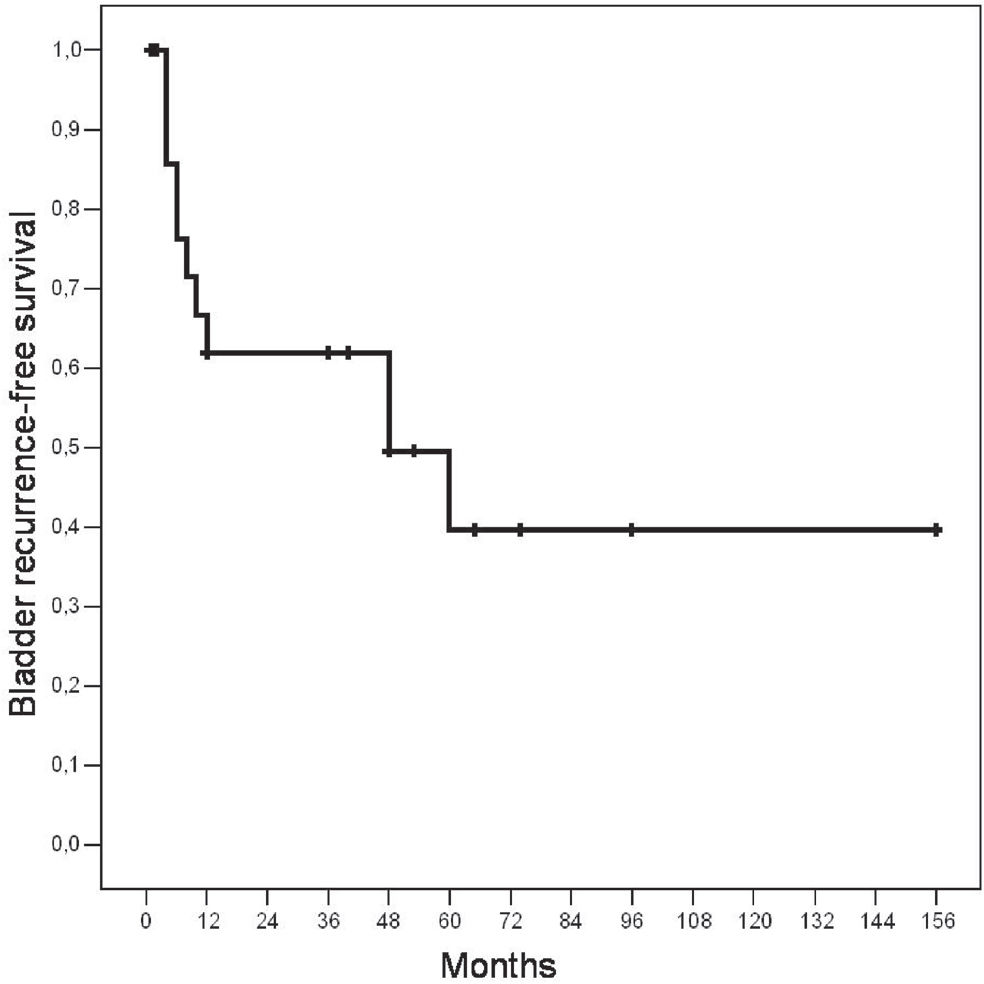
RESULTSEighty-four percent of patients were male, and macroscopic hematuria was the most common clinical presentation. The majority of cases (56%) were infiltrative (T2–T3) and high-grade (76%) tumors. Synchronous or metachronous bladder tumors were found in 72% of cases. Five (20%) patients had a history of bladder tumor before the diagnosis of upper urinary tract transitional cell carcinomas. The mean follow-up period was 36 months (range: 1.5 to 156). During the follow-up period, eleven (44%) patients developed bladder tumors. After five years, the probability of being free of bladder tumor recurrence was 40%. No pathological variable was predictive for bladder tumor recurrence. Four patients presented disease recurrence outside the urinary tract.
CONCLUSIONSThe presence of metachronous bladder tumors is more often observed after the diagnosis of upper urinary tract transitional cell carcinomas. All of these patients should undergo rigorous follow-up during the postoperative period. Only patients with infiltrative and high-grade tumors developed metastases outside the urinary tract.
Upper urinary tract transitional cell carcinomas (UUT-TCC) are rare lesions. They account for 5% of all urothelial tumors and 10% of all renal tumors.1 As North American statistics estimate that there will be 38,890 new cases of kidney and renal pelvis tumors in 2006, this represents only about 3,889 new cases of renal pelvis tumors during the current year.2 Primary ureteral tumors are even rarer and are found 3 to 4 times less frequently than renal pelvis tumors.3 Nephroureterectomy with bladder cuff removal is considered the standard treatment for UUTTCC;4 however, these tumors often behave aggressively, and local failure rates are high even after radical surgical treatment.1 Due to its inherent rarity, most of the clinical-pathological characteristics of UUTTCC are not well known, and a better understanding of these characteristics would allow for earlier diagnosis and more efficient treatment for the patients. One of the most important characteristics of surgically-treated urothelial carcinomas is the ongoing risk of developing tumors in the remnant urothelium during the follow-up period.5,6 In addition, the best follow-up protocol has not, thus far, been defined.
In the present study, the authors describe the main clinicopathological characteristics of patients with UUTTCC who are treated surgically, and analyze the occurrence of synchronous and metachronous bladder tumors as well as the development of metastases outside the urinary tract.
MATERIALS AND METHODSThe study comprised a retrospective analysis of 25 patients with UUTTCC treated between February 1994 and August 2006. Patients with unavailable clinical and pathologic data, as well as patients with other histologic types of tumors, were excluded from the analysis. All information was obtained by a review of the medical records.
Regarding preoperative image analysis, an ultrasound (USG) of the urinary tract was performed in 17 (68%) cases, excretory urography in 14 (56%) cases, and abdominal computed tomography (CT) in 22 (88%) cases. A retrograde pyelogram was performed in eight (32%) patients at the time of surgery to clarify the diagnosis.
All surgical procedures were performed by only one surgeon (M.S.). Twenty-one patients (84%) underwent a classic nephroureterectomy with bladder cuff removal. Four patients underwent renal sparing surgery, including two patients of advanced age who had small primary ureteral tumors and underwent partial ureterectomy, one patient with a right renal agenesis who underwent an inferior pielectomy, and one patient that presented with bilateral UUTTCC and was found to have panurothelial disease. This patient underwent a cystectomy with bilateral ureterectomy. Dissection of the regional lymph nodes was performed only in patients who had enlarged nodes based on preoperative image analysis or intraoperative examination. The follow-up schedule was the same for all patients; they were seen every four months for the first year and semiannually thereafter. Office visits consisted of physical examination, serum chemistry, and urinalysis with urinary cytology. Diagnostic CT imaging of the abdomen and pelvis was performed on the same schedule, and cystoscopy was performed on a semiannual basis.
The variables analyzed were: patient age, gender, and clinical presentation; diagnostic methods; pathologic characteristics at the primary site of the tumor (pelvis or ureter); tumor stage and grade; and the presence of carcinoma in situ, microvascular invasion (MVI), and squamous differentiation. We also analyzed the occurrence of synchronous or metachronous bladder tumors and the characteristics of these lesions. All superficial bladder tumors were treated with intravesical Bacillus Calmette-Guerin vaccine if they had one of following characteristics: high grade, stage of T1, recurrence, multiple lesions, or association with carcinoma in situ.
The pathological stage was defined according to the UICC classification,7 and tumor grade was based on the 2004 World Health Organization system.8 All pathologic analyses were performed by a single pathologist (K.R.L.). The Kaplan-Meier method and the Log Rank test were used for analysis of bladder recurrence-free survival. Statistical significance was set as p ≤ 0.05. Statistical analysis was performed using SPSS 12.0 for Windows.
RESULTSThe mean patient age was 64.4 years (range: 39 to 85 years). There were 21 men and 4 women. At the time of diagnosis, 20 (80%) patients presented with macroscopic hematuria as the main complaint. Among the remaining five patients, one had urinary retention due to an enlarged prostate, two had irritative lower urinary tract symptoms, one had lumbar pain and one had only microscopic hematuria. Microscopic hematuria was found in all cases. Overall, flank pain was present in four (16%) patients. The length of time from the beginning of symptoms to definitive diagnosis varied from two days to nine months among the patients. Regarding image analysis, the USG of the urinary tract suggested the correct diagnosis in 5/17 (29.4%) cases, the excretory urography in 14/14 (100%) cases, and the abdominal CT in 19/22 (86.3%) cases.
Table 1 describes the main clinicopathological findings for the 25 UUTTCC. In 21 (84%) cases, tumors were located in the renal pelvis. Tumors were located on the right side in 14 (56%) cases. There was only one synchronous ureteral tumor and no metachronous tumors in the contralateral upper tract. The majority of cases (56%) were infiltrative (T2–T3) and high-grade (76%) tumors. In situ carcinoma was found in 12%, MVI in 36%, and squamous differentiation in 16% of cases. No patient presented with positive lymph nodes.
Clinicopathological characteristics of the 25 UUT-TCC
| Median Age (Min – Max) | 64 (39 – 85) |
|---|---|
| Gender | |
| Male | 21 (84.0%) |
| Female | 4 (16.0%) |
| Clinical findings | |
| Macroscopic hematuria | 20 (80%) |
| Flank pain | 4 (16%) |
| No symptoms | 1 (4%) |
| Side of tumor | |
| Right | 14 (56%) |
| Left | 11 (44%) |
| Primary site | |
| Pelvis | 21 (84%) |
| Ureter | 4 (16%) |
| Stage | |
| Ta | 2 (8.0%) |
| T1 | 9 (36.0%) |
| T2 | 3 (12.0%) |
| T3 | 11 (44.0%) |
| Grade | |
| Low | 6 (24.0%) |
| High | 19 (76.0%) |
| Squamous differentiation | |
| Yes | 4 (16.0%) |
| No | 21 (84.0%) |
| MVI | |
| Yes | 9 (36.0%) |
| No | 16 (64.0%) |
| Carcinoma in situ | |
| Yes | 3 (12.0%) |
| No | 22 (88.0%) |
Synchronous or metachronous bladder tumors were found in 72% of cases. Five (20%) patients had a history of bladder tumors before the diagnosis of UUTTCC. The length of time from the diagnosis of the first bladder tumor to the diagnosis of UUTTCC varied from 10 months to 12 years (mean of 72 months). All of these cases presented superficial (Ta-T1) grade 1 to 3 bladder carcinomas. Two patients were found to have bladder tumors at the time of UUTTCC diagnosis: one had a Ta grade 3 tumor and the other had a T2 grade 4 tumor. The latter patient was an 85-year old man who was not considered for radical cystectomy due to his advanced age.
The mean follow-up period was 36 months (range: 1.5 to 156 months). During the follow-up period, eleven (44%) patients developed bladder tumors. The mean length of time for diagnosis of bladder tumor was 19 months (4 to 60 months). The probability of being free of bladder tumor recurrence was 40% when patients were followed-up for at least five years (Figure 1). All of the cases but one were superficial (Ta-T1) grade 1 to 3 tumors, and only one had an associated in situ carcinoma. Using the Log-Rank test, we found that no clinicopathological variable was associated with an increased risk of bladder tumor recurrence. Three patients underwent radical cystectomy: one 12 years before the diagnosis of UUTTCC, one simultaneously with the diagnosis of UUTTCC (described above), and one 15 months after a right nephroureterectomy for a T1 grade 3 bladder tumor (Table 2).
Characteristics of synchronous and metachronous bladder tumors
| Characteristics | n |
|---|---|
| Patients with bladder tumors | 18 (72%) |
| Occurrence of the bladder tumor | |
| Before | 5 (mean 72 months earlier) |
| Simultaneous | 2 |
| After | 11 (mean 19 months later) |
| Pathological characteristics of the bladder tumors | |
| Stage | |
| Ta | 12 |
| T1 | 5 |
| T2 | 1 |
| Grade | |
| Low | 9 |
| High | 9 |
| Carcinoma in situ | 1 |
| Cystectomy | 3 |
| Before | 1 (12 years earlier) |
| Simultaneous | 1 |
| After | 1 (15 months later) |
Four patients presented disease recurrence outside the urinary tract. Two patients developed pelvic masses 10 and 30 months after treatment, respectively, and two developed pulmonary metastases 8 and 33 months after treatment, respectively. The first two patients had presented a T1 grade 3 and T3 grade 3 UUTTCC, and the pelvic masses were surgically removed. The patients underwent adjuvant chemotherapy. The latter two patients presented with infiltrative grade 4 UUTTCC. Pulmonary metastases were treated with chemotherapy, but the patients died a few months later.
DISCUSSIONIn the present study, the authors describe important clinicopathological characteristics of UUTTCC. Eighty-four percent of patients were male, and macroscopic hematuria was the most common clinical presentation. The most accurate preoperative image analysis was excretory urography, followed by CT and USG. Currently, multidetector CT scans are more accurate than the older ones used in this series; thus, CT will likely reach the same accuracy as excretory urography in the near future.9 Most of the tumors in the present series showed aggressive pathological characteristics, as 56% were infiltrative (T2–T3) and 76% were high-grade tumors. In the series assessed by Park et al,1 86 patients with UUTTCC were analyzed and hematuria and flank pain were found in 77.9% and 16.3% of cases, respectively. Infiltrative tumors comprised 57% of cases, and 93% of cases were grade 2 or 3 tumors. In the present study, pelvis tumors were five times more common than ureteral tumors. All four cases of ureteral tumors were superficial lesions, and two of them were low-grade lesions. Because we examined only four primary ureteral tumors, an accurate comparison with pelvis tumors was not possible. Usually, cases of ureteral tumors present higher rates of infiltrative and high-grade lesions and show significantly lower disease-free survival than those of pelvis tumors.1 These characteristics are probably due to the thin muscle layer of the renal pelvis and ureter.
MVI and squamous differentiation are two less well described pathological characteristics of UUTTCC. Recently, Kikuchi et al10 reported that in multivariate analysis of MVI, pathological stage and tumor grade were independent predictors of disease-specific survival. They stratified patients into three risk groups based on these characteristics. With regard to squamous differentiation, Antunes et al. showed that this characteristic is an independent prognostic factor for bladder cancer-specific survival11. In the present study, MVI and squamous differentiation were present in 36% and 16% of cases, respectively.
The occurrence of synchronous or metachronous bladder tumors was found in 72% of cases. Five patients had a history of bladder tumors prior to the diagnosis of UUTTCC. All cases were superficial bladder tumors (Ta - T1) and the mean time to diagnosis of UUTTCC was 72 months. The proportion of patients who develop upper urinary tract tumors after the diagnosis and treatment of superficial bladder tumors has been reported as only 0.002% to 2.4% in one series following surveillance intervals of 5 to 13 years.12 According to Canales et al.,6 the risk of upper urinary tract recurrence is increased even in patients with Ta bladder cancer. A review of 375 patients who underwent resection of a stage Ta tumor with a median follow-up of 6 years showed that UUTTCC had developed in 13 patients (3.4%) an average of 22 months after their initial bladder tumor. A high-risk group consisting of patients who had two or more bladder recurrences within 12 months of each other was at a 4.5-fold greater risk of UUTTCC. A low incidence of UUTTCC was also observed for patients submitted to radical cystectomy according to Sanderson et al., who examined more than 1000 patients.13
The majority of cases in the present series developed bladder tumors after treatment for UUTTCC, all of which were also superficial bladder lesions. Among the 11 cases of UUTTCC who developed bladder tumors, six were infiltrative and eight were high-grade lesions. No clinicopathological variable was associated with an increased risk of bladder recurrence. In the series assessed by Holmang and Johansson,14 bladder cancer was present in 83% of patients with bilateral UUTTCC compared to 31% among those with unilateral UUTTCC. Interestingly, Raman et al. found that more than 90% of cases were superficial bladder cancers, and the majority of these were low-grade. In this series, the only predictor of the subsequent development of a bladder tumor was a history of bladder cancer.15 This study is part of the general trend in our service to survey diagnosis and risk factors of urologic neoplasias.16
No patient in the present series developed metachronous tumors in the contralateral upper urinary tract during the follow-up period. The risk of metachronous UUTTCC among 768 patients with UUTTCC diagnosed in a 28-year period was 3.1% after a median follow-up of 46 months. The projected incidence after initial UUTTCC diagnosis was 2.7%, 5.8% and 6.5% at 5, 10 and 15 years, respectively14. Only one patient in the present series presented bilateral disease. This patient was a 59-year-old female with a history of recurrent superficial bladder cancer. Since the initial diagnosis twelve years earlier, she presented 12 recurrences in the bladder, all of which were treated with transurethral resection and adjuvant therapy. Pathology samples had always shown Ta grade 1 transitional cell carcinoma. At the last recurrence, she presented a papillary grade 3 transitional cell carcinoma. At this time, an intravenous urography and ureteroscopy revealed lesions in both ureters. The patient underwent a radical cystectomy with total right ureterectomy and distal left ureterectomy. Orthotopic ileal neobladder reconstruction was performed, with the distal ileum detubulized and rearranged into a U shape. Both ends of the “U” shaped ileum were kept long enough to perform a pyelo-ileal anastomosis and a proximal uretero-ileal anastomosis at the right and left sides, respectively17.
Three patients underwent radical cystectomy during the study period: one 12 years before UUTTCC diagnosis, one simultaneously with UUTTCC diagnosis (described above), and the other 15 months after a right nephroureterectomy. In the series performed by Akkad et al.,5 the risk of developing an UUTTCC after radical cystectomy was 4.7% after a 56 month follow-up period. All patients who had secondary UUTTCC had undergone cystectomy for multifocal or recurrent TCC.
All four patients who developed metastases outside the urinary tract had high-grade tumors, and three of them had infiltrative (T2–T3) tumors. An analysis of the prognostic factors for 5-year recurrence-free survival in 72 patients treated for UUTTCC revealed that stage, grade and tumor location in the urothelium were independent prognostic variables based on multivariate analysis.18 Based on these findings, one might consider treating low-grade superficial tumors conservatively. However, we cannot comment on this issue due to our small sample size. Furthermore, this remains a controversial issue in the literature, with higher recurrence rates and lower cancer-specific survival with conservative treatment.19
Finally, it is important to address the retrospective design of our study, as well as the small sample size due to the rarity of this cancer. However, we can conclude that most UUT-TCC are infiltrative and high-grade lesions. The presence of metachronous bladder tumors are more often observed after UUTTCC diagnosis, and the great majority of these tumors are superficial lesions that can appear several years after the diagnosis of UUTTCC. No clinicopathological variable is predictive of bladder recurrence; thus, all of these patients should undergo rigorous follow-up with urinary cytology, USG and cystoscopic examinations during the postoperative period. In our study, only patients with infiltrative and high-grade tumors developed metastases outside the urinary tract.