To evaluate the anti-arthritic potential of the plant Justicia gendarussa using two different rat models.
MATERIALS AND METHOD:The anti-arthritic potential of the alcoholic extract of the plant Justicia gendarussa was evaluated using the Freund’s adjuvant-induced and collagen-induced arthritic rat models. The rats were treated with the ethanolic extract of Justicia gendarussa and with standard aspirin.
RESULTS:The ethanolic extract of Justicia gendarussa showed significant anti-arthritic activity that was statistically similar to that of aspirin. Our results suggest that the alcoholic extract of Justicia gendarussa exhibits significant anti-arthritic potential.
Rheumatoid arthritis (RA) is a ravaging inflammatory and autoimmune illness that affects the joints.1 This disease also affects the tissues surrounding the joints such as skin, blood vessels, and muscles.2 Although various drugs have been used to control RA, there are numerous reports regarding the side effects of these drugs. A range of newer drugs called TNF blockers have been linked to a condition called leukocytoclastic vasculitis, or LCV.3–5 TNF blockers, specifically Humira and Remicade, reportedly increase the risk of cancer and serious infections3. As a consequence, researchers are now searching for alternatives therapeutics. As part of this search, significant attention has been paid to the plant based drugs that are used in traditional medicine because these drugs elicit few side effects and are inexpensive.5 One such plant that is widely used in Indian and Chinese traditional medicines but that has not been studied in a well-controlled experimental trial to date, is the species Justicia gendarussa (J. gendarussa).
Justicia gendarussa Burm F. (Family: Acanthaceae) is a shade-loving, quick-growing, evergreen plant mostly found in moist areas. It is believed to be native to China and is distributed widely across India, Sri Lanka, and Malaysia. In Indian and Chinese traditional medicine, the leaf of the plant is recommended to treat ailments such as fever, hemiplegia, rheumatism, arthritis, headache, earache, muscle pain, respiratory disorders, and digestive trouble.6,7 However, to our knowledge, there are no published scientific studies on the anti-arthritic activities of the leaves of J. gendarussa or its potential toxicity. Therefore, the objective of this study is to examine the anti-arthritic potential and toxicity of the ethanolic leaf extract of this plant.
MATERIALS AND METHODSPlant materialJ. gendarussa leaves were collected from Udupi (Karnataka district, India) in August, 2006. The plant was identified by the Department of Botany, Mahatma Gandhi Memorial College, Manipal, India, and the voucher specimen was kept in the institutional herbarium for future reference. Two kilograms of shade-dried leaves was blended to a fine powder and extracted with ethanol (95%) using the soxhlet method.8 The extract was concentrated by distillation under reduced pressure and evaporated to dryness. The total yield of the dried powder was 65 g.
Experimental animalsMale albino Wistar rats weighing approximately 180–200 g were used for the studies. The animals were housed in cages under standard laboratory conditions (12/12 hour light/dark cycle at 25 ± 5ºC). The rats were fed a commercial rat diet and water ad libitum and were divided into groups of six. All experiments were conducted following the ethical guidelines for the use of animals in research at our institution.
Acute toxicity testA group of six rats was administered the plant extract in graded doses of 0.25, 0.5, 1.0, 1.5, and 2.0 g/kg rat. Rats were continuously observed for mortality and behavioral responses for 48 hr and once daily thereafter until the 14th day. Dose selection was performed by taking 1/10th of the lethal dose. Ld50 as obtained from this experiment was 1000 mg/kg rat. The dose of the J. gendarussa extract selected for our experiment was therefore 100 mg/kg rat. Aspirin was used as a standard drug against which to compare the efficacy of J. gendarussa. The dose of aspirin was selected as 360 mg/kg rat by using a human-rat conversion factor.
Induction of arthritisArthritis was induced by injecting the rats with Freund’s Complete Adjuvant (FCA DIVISION) or Bovine type II Collagen (COLLAGEN DIVISION). In the first method, 0.5 mL of FCA containing 10 mg of dry heat-killed Mycobacterium butyricum/mL in sterile paraffin oil (Difco Laboratories, Detroit, MI) was injected into the plantar surface of the left hind foot of the animal.9,10
In the second method, 0.1 mL of collagen emulsified with Incomplete Freund’s adjuvant (IFA) was injected into the left hind foot.11 The degree of hind paw swelling in each animal was determined using a plethysmograph. A qualitative scoring system was used to assess the severity of paw inflammation. The absence of any visible swelling was given a score of 0, mild redness and swelling of individual digits, regardless of the number of affected digits was given a score of 1, moderate redness and swelling of the ankle was given a score of 2, and severe redness and swelling of the entire paw including the digits was given a score of 3. Rats with a score of 3 were considered arthritic and were used for subsequent experiments.
Experimental designIn each of the two divisions (FCA and COLLAGEN), animals were subdivided into four groups of 6 rats each, as illustrated in Figure 1: FCA Group I – Normal rats administered gum acacia (gum acacia was used as vehicle); FCA Group II – Arthritic rats administered gum acacia; FCA Group III - Arthritic rats administered Aspirin; FCA Group IV - Arthritic rats administered the leaf extract of J. gendarussa. COLLAGEN Group I – Normal rats administered gum acacia; COLLAGEN Group II – Arthritic rats administered gum acacia; COLLAGEN Group III - Arthritic rats administered Aspirin; COLLAGEN Group IV - Arthritic rats administered the leaf extract of J. gendarussa. J. gendarussa and Aspirin were administered starting on the 20th day following the induction of arthritis and continued for 20 days. During the 20 days of treatment, the paw volumes of the animals were recorded at regular intervals. At the end of the 20th day, animals were euthanized by cervical dislocation. Serum samples were collected for further biochemical assays.
Phytochemical screeningThe ethanolic extract of J. gendarussa was tested using standard procedures to identify the presence of sterols and flavonoids. Compounds separated using preparative paper chromatography were further assessed using the Liebermann-Burchard and Shinoda’s tests to identify the presence of sterols and flavonoids, respectively.
Biochemical assaysHemoglobin (Hb) content was estimated by the method of Drabkin and Austin.12 Red blood cell (RBC) and white blood cell (WBC) counts were estimated according to the method of Chesbrough and McArthur13 in an improved Neubauer chamber. Estimations of erythrocyte sedimentation rates (ESR) were carried out by the method of Westergren.14 For the estimation of copper levels, the Colorimetric Bathocuproin disulfonate method of Zak and Landers15 was used. C-reactive protein levels were estimated using the ELISA kit obtained from Alpha Diagnostics Intl., USA.
Statistical analysisThe results were analyzed using one way Analysis Of Variance followed by the Bonferroni test. The values are expressed as means ± SEM.
RESULTSAcute toxicity tests showed an Ld50 of 1000 mg/kg with the leaf extract. We noted no behavioral or morphological changes when 1/10th of the Ld50 was administered to a group of 6 rats for 40 days. Therefore, this dose was taken as the experimental treatment dose for the arthritic rats.
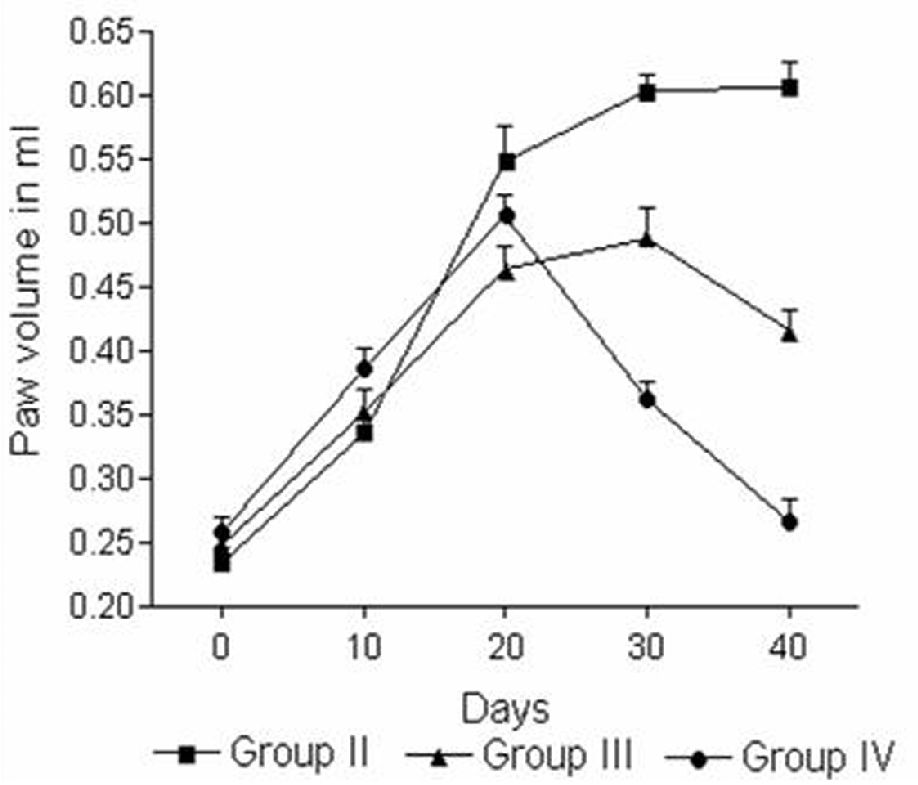
Paw volumeIn arthritic rats, the paw volume reached its maximum on the 20th day in both arthritic models. The paw maintained its inflamed condition for the next 20 days in Group II rats. A significant reduction in paw volume was observed in the J. gendarussa-treated (group IV) and aspirin-treated (group III) rats as compared to group II. The results are illustrated in Figures 2 and 3.
Changes in the paw volumes of rats in group II (FCA-induced arthritic control), group III (FCA-induced arthritic rats treated with Aspirin), and group IV (FCA-induced arthritic rats treated with J. gendarussa extract). Values are mean ± SEM; n = 6. *P< .001 for the comparison of groups III and IV with Group II on the 40th day
Changes in the paw volumes of rats in group II (collagen-induced arthritic control), group III (collagen-induced arthritic rats treated with Aspirin), and group IV (collagen-induced arthritic rats treated with J. gendarussa extract). Values are mean ± SEM; n = 6. *P< .001 for the comparison of groups III and IV with Group II on the 40th day
Tables 1 (FCA) and 2 (COLLAGEN) show hematological parameters such as Hb, RBC count, WBC count, ESR, serum copper levels, and C-reactive protein levels for both normal and experimental rats. We observed a similar trend in the hematological parameters from rats in the FCA and Collagen groups. A significant decrease in the levels of RBC and Hb was observed in arthritic rats (group II) when compared to normal rats (group I). Administration of J. gendarussa extract to arthritic rats (group IV) enhanced the levels of Hb and RBC to near normal levels. The increases in WBC count, ESR, serum C-reactive protein level, and serum copper level were significantly suppressed in the extract-administered arthritic group (group IV) (Table 2).
Effects of Aspirin (group III) and J. gendarussa extract (group IV) on hematological parameters in normal and FCA-induced arthritic rats. Values are mean ± SEM; n = 6
| Parameter | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|
| Hb (g/dL) | 12.25 ± 0.21 | 9.00 ± 0.18a | 9.67 ± 0.25 | 11.75 ± 0.21c |
| RBC (x106/mm3) | 4.48 ± 0.01 | 3.76 ± 0.02a | 3.78 ± 0.01 | 4.14 ± 0.17c |
| WBC (x103/mm3) | 7.34 ± 0.14 | 17.43 ± 0.16a | 12.78 ± 0.27b | 8.88 ± 0.18c |
| ESR | 3.33 ± 0.33 | 10.67 ± 0.42a | 10.50 ± 0.43 | 5.17 ± 0.31c |
| CRP (μg/mL) | 172.9 ± 3.47 | 425.7 ± 9.62a | 254.2 ± 3.95b | 285.0 ± 3.14c |
| Copper (μg/mL) | 103.2 ± 7.27 | 186.1 ± 3.25a | 138.1 ± 5.01b | 124.3 ± 6.26c |
Effect of Aspirin (group III) and J. gendarussa extract (group IV) on hematological parameters in normal and collagen-induced arthritic rats. Values are mean ± SEM; n = 6
| Parameter | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|
| Hb (g/dL) | 12.67 ± 0.21 | 8.92 ± 0.15a | 9.17 ± 0.28 | 11.17 ± 0.28c |
| RBC (x106/mm3) | 4.78 ± 0.06 | 3.52 ± 0.09a | 3.41 ± 0.15 | 4.40 ± 0.12c |
| WBC (x103/mm3) | 6.38 ± 0.16 | 4.23 ± 0.25a | 8.24 ± 0.22b | 7.48 ± 0.13c |
| ESR | 4.17 ± 0.31 | 11.83 ± 0.31a | 11.67 ± 0.67 | 5.83 ± 0.40c |
| CRP (μg/mL) | 76.87 ± 2.43 | 411.7 ± 10.59a | 288.3 ± 5.97b | 111.4 ± 0.69c |
| Copper (μg/mL) | 118.5 ± 7.27 | 187.8 ± 4.68a | 142.0 ± 4.63b | 127.3 ± 4.41c |
Freund’s complete adjuvant (FCA)-induced arthritis and collagen-induced arthritis are two models that have been extensively used to study the pathogenesis of rheumatoid arthritis for therapeutics testing.10 Collagen-induced arthritis (CIA) is an experimental model that shares several clinical and pathological features with rheumatoid arthritis (RA). Scientists have established the importance of T cells in the pathogenesis of CIA and the role of cytokines in the progression of arthritis.16,17
Paw swelling is one of the major factors in assessing the degree of inflammation and curative efficacy of drugs.18 Here, the J. gendarussa-treated rats showed paw edema inhibition of 43% in the FCA-induced arthritic model and 47% in the collagen-induced arthritic model. By comparison, aspirin-treated rats showed paw edema inhibition rates of 26% and 38% in the FCA-induced arthritic model and the collagen-induced arthritic model, respectively.
Anemia is commonly noted in patients with chronic arthritis.19 The two most common explanations are gastrointestinal blood loss due to arthritis medications and bone marrow changes in patients with inflammatory arthritis, which prevents the release of iron for incorporation into red blood cells.20,21 In our study, arthritic rats (group II) showed a reduced RBC count, reduced Hb levels, and an increased erythrocyte sedimentation rate (ESR). All these symptoms indicate an anemic condition, which persisted in the Aspirin-treated group (group III). The J. gendarussa-treated group (group IV) showed a significant recovery from the induced anemia. The anemic condition persisted in the Aspirin-treated group as indicated by the RBC count and Hb level. An indicator of infectious and inflammatory diseases,22 the WBC count was increased in arthritic rats. The migration of leukocytes to the inflamed area was significantly suppressed by this plant extract, as indicated by the significant decrease in the WBC count.
C-reactive protein is a member of the class of acute phase reactants - its levels rise dramatically during inflammatory processes.23 The concentration of C-reactive protein was found to be significantly reduced in the plant-treated as well as the Aspirin-treated groups.
Ceruloplasmin, an enzyme synthesized in the liver, contains 8 atoms of copper in its structure. Free copper ions are powerful catalysts of free radical damage. By binding copper, ceruloplasmin prevents free copper ions from catalyzing oxidative damage.24 Increased copper ion concentrations indicate the extent of an inflammatory condition.25 The concentration of serum copper was measured in both normal and arthritic rats. The arthritic rats exhibited significantly elevated copper levels, which were suppressed in J. gendarussa- and aspirin-treated rats.
Phytochemical analyses of the J. gendarussa leaf extract showed the presence of the flavonoids vitexin and apigenin. J. gendarussa extract has been reported to contain -sitosterols, alkaloids, reducing sugars, as well as unidentified sterols.26 Apigenin has been reported to exert anti-inflammatory effects such as lowering oxidative stress and forestalling the expression of several inflammatory factors.27 There are also reports on the flavonoid vitexin as a potent anti-inflammatory agent; vitexin may exert its anti-inflammatory activity by inhibiting the 5-lipoxygenase pathway, which together with the COX-2 pathway, is very important in producing and maintaining inflammation.28
Taken together, our results strongly support the anti-arthritic potential of the plant J. gendarussa and its use in traditional medicine. As mentioned previously, compounds such as flavonoids and β-sitosterols are well known for their anti-inflammatory activities. The presence of these compounds in the J. gendarussa leaf extract may explain the anti-arthritic properties of this plant. Our next goal is to isolate and identify the compounds responsible for the anti-arthritic potential of this plant.