Despite the recent success regarding the transplantation of tissue-engineered airways, the mechanical properties of these grafts are not well understood. Mechanical assessment of a tissue-engineered airway graft before implantation may be used in the future as a predictor of function. The aim of this preliminary work was to develop a noninvasive image-processing environment for the assessment of airway mechanics.
METHOD:Decellularized, recellularized and normal tracheas (groups DECEL, RECEL, and CONTROL, respectively) immersed in Krebs-Henseleit solution were ventilated by a small-animal ventilator connected to a Fleisch pneumotachograph and two pressure transducers (differential and gauge). A camera connected to a stereomicroscope captured images of the pulsation of the trachea before instillation of saline solution and after instillation of Krebs-Henseleit solution, followed by instillation with Krebs-Henseleit with methacholine 0.1 M (protocols A, K and KMCh, respectively). The data were post-processed with computer software and statistical comparisons between groups and protocols were performed.
RESULTS:There were statistically significant variations in the image measurements of the medial region of the trachea between the groups (two-way analysis of variance [ANOVA], p<0.01) and of the proximal region between the groups and protocols (two-way ANOVA, p<0.01).
CONCLUSIONS:The technique developed in this study is an innovative method for performing a mechanical assessment of engineered tracheal grafts that will enable evaluation of the viscoelastic properties of neo-tracheas prior to transplantation.
Tracheal organic substitutes have been investigated clinically and experimentally. The creation of a neo-trachea can be achieved using hybrid compounds that combine autologous tracheal tissue with a collagen framework (1,2). The trachea can be tissue-engineered (3), consisting of a decellularized allograft repopulated with recipient cells in situ (4). Among the tracheal substitutes developed, those generated by tissue engineering (3,5) and allografts repopulated with the recipient's own cells in situ have been used in clinical practice (4,6).
Despite both experimental and clinical successes achieved with the transplantation of tissue-engineered airways, the mechanical properties of the grafts and the eventual changes in their structure have not yet been studied. We hypothesize that the mechanical properties of a tissue-engineered airway graft before implantation may be used as a predictor of its function and, therefore, of the outcome following airway transplantation.
This preliminary study focused on the development of a noninvasive image-processing environment for the assessment of airway mechanics that enables measurement of the diameter, pressure and flow variation of tracheal grafts.
MATERIALS AND METHODSThe environment used in this study for the measurement of airway respiratory mechanics has been reported elsewhere (7). Briefly, the environment consists of a small-animal ventilator (SAV) (Harvard 683, Harvard Apparatus, USA) serially connected to a Fleisch pneumotachograph (000, Hugo Sachs, Switzerland), a differential pressure transducer (HCXPM005D6V, Sensortechnics, USA) and a gauge pressure transducer (FPM 07PG, Fujikura, Japan). Tracheas were connected to the SAV and were kept immersed in Krebs-Henseleit solution in an open-top polyurethane conditioner (Figure1). A camera (SDC 415, Samsung, South Korea) connected to a stereomicroscope (SZ61, Olympus, Japan) captured images of the pulsation of the tracheas. The data and video acquisition systems were controlled using a virtual instrumentation environment (LabVIEW 2012, National Instruments, USA). The data collected by the transducers and video recordings were post-processed with software developed in an environment for numerical computation (Matlab, MathWorks, USA).
Environment used for the measurement of airway respiratory mechanics using image processing. (a) Trachea immersed in Krebs-Henseleit solution in an open-top polyurethane conditioner. (b) Overview of the stereomicroscope, airway conditioner, pressure transducers, Fleisch pneumotachograph, and SAV.
The animals received humane care according to the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources – National Academy of Sciences, Washington, D.C., 1996). The experimental protocol was approved by the ethics committee (CAPPesq 0908/07) of the Hospital das Clínicas da Faculdade de Medicina da Unversidade de São Paulo.
The experimental design included 3 groups: CONTROL (normal trachea), DECEL (decellularized trachea) and RECEL (recellularized trachea). In the CONTROL group, tracheas from 5 Sprague-Dawley rats (270 to 292 g) were harvested and incubated for 40 minutes in Krebs-Henseleit solution at 35°C. The tracheas were then placed in the conditioner to start the experiments. In the DECEL group, tracheas were harvested from 5 Wistar rats (300 to 350 g). The decellularization process followed a method validated and described previously (8), followed by storage at 4°C to 7°C in 70% ethyl alcohol until use in the experiments. In the RECEL group, the same decellularization process was performed using tracheas from 5 Wistar rats (300 to 350 g). Recellularization employed mesenchymal stem cells (MSCs) obtained from the adipose tissue of the donor animals sacrificed to obtain the tracheas. Briefly, the harvested adipose tissue was submitted to enzymatic digestion to obtain the MSCs, placed in a collagenase type I enzymatic solution (Invitrogen, USA) and stored in 5% CO2 at 37°C for 15 hours. After inactivation with fetal bovine serum (FBS), the material was centrifuged (1200 rpm, 10 minutes) and the pellet that formed was resuspended in serum replacement (Invitrogen, USA). After the MSCs were obtained, cell viability and number were determined before proceeding with cell plating and culture. After amplification, characterization of the expression of the markers CD90, CD45 and CD34 was performed by flow cytometry, followed by differentiation to establish 3 cell lines: adipogenic, chondrogenic and osteogenic. For chondrogenic differentiation, the protocol described by Moroz (9) and modified by Silva (10) was used. The microencapsulation procedure was executed in concentrated platelet gel to ensure the necessary 3D culture structure for chondrogenic differentiation. The cells used for recellularization were maintained in DMEM until the time of the experiments. Prior to the experiments themselves, the tracheas were incubated in Krebs-Henseleit solution maintained at 35°C for 40 minutes.
Each trachea was immersed in the conditioner, which contained Krebs-Henseleit solution at 35°C, exposing the outer surface of the trachea to the solution, whereas the tracheal lumen was kept dry. The trachea was connected to the conditioner by a proximal metallic tube (14 gauge) connecting the trachea to the ventilator and by a distal tube (14 gauge) connecting the trachea to the outlet. Such a system allows air to flow from the SAV (tidal volume of 3 mL/cycle; frequency of 80 breaths/minute) through the trachea and an outlet catheter with a known resistance to produce tracheal pulsation.
For the first measurements, the SAV was started and the measurements were obtained over 3 minutes (protocol A). The SAV was then stopped and the conditioner was disconnected from the ventilator. Krebs-Henseleit solution was injected into the tracheal lumen via the proximal opening and was allowed to sit for 60 seconds. The solution was then removed and the conditioner was once again connected to the SAV. Measurements were performed for 3 more minutes under ventilation (protocol K). The same procedure was performed using Krebs-Henseleit solution with methacholine 0.1 M (protocol KMCh). In the DECEL and RECEL groups, the measurements were performed for one minute only.
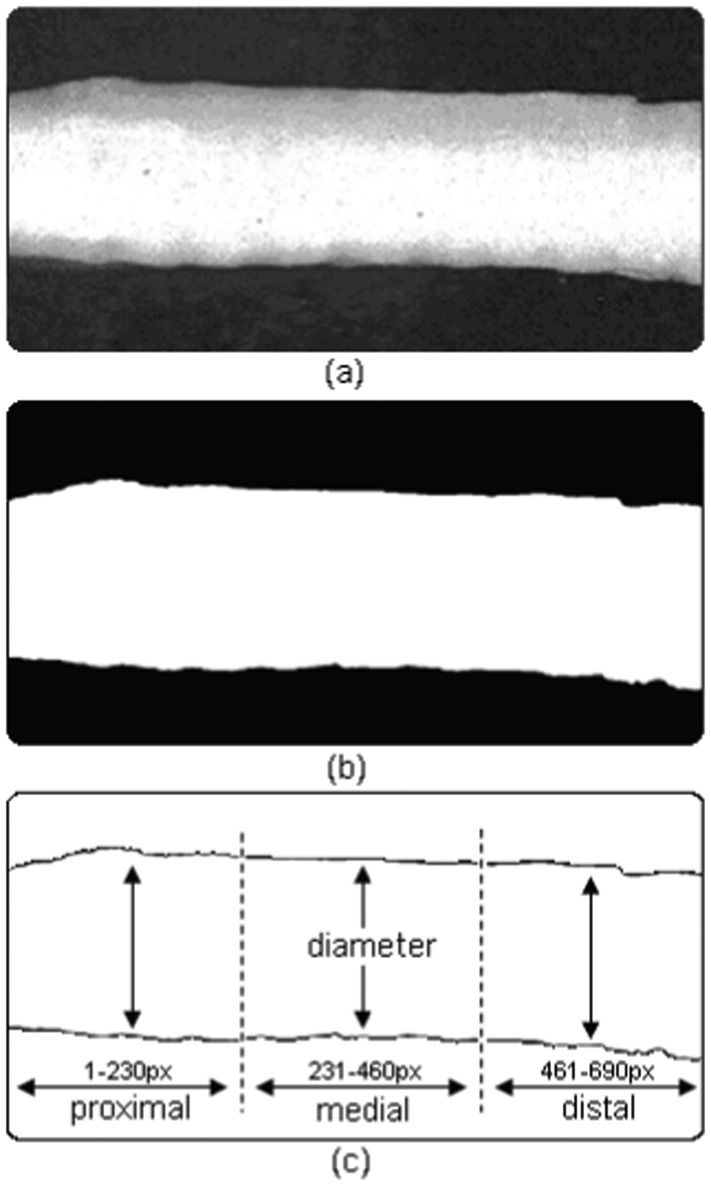
The data at the peak of the respiratory cycle were obtained for each cycle. The pulsation of the trachea was measured in three tracheal segments: proximal (next to the outward tube), medial and distal (next to the inward tube).
Statistical analyses of the measurements of the images were performed in a statistical environment (Prism version 5.03 for Windows, GraphPad Software, USA). To determine significant differences between the groups (CONTROL, DECEL and RECEL) and/or between the protocols (A, K and KMCh) in the measurements of the segments, two-way analysis of variance (ANOVA) with repeated measures for one of the factors was performed (p<0.05). The Kolmogorov-Smirnov test was conducted to verify adherence to the normal curve. A descriptive analysis was performed using spreadsheet software (Excel 2010, Microsoft, USA).
RESULTSFigure2 depicts an image of a trachea in the CONTROL group, showing the result of image segmentation using the Otsu technique for determining the optimal threshold (11) and showing how the percentage change in the tracheal diameter was obtained. The percentage change in the proximal pulsation represents the mean measurement of percentage changes in 1-230 pixels in the captured image. The medial and distal pulsations represent the mean of pixels 231-460 and 461-690, respectively. Each pulsation represents an insufflation caused by the SAV. The results of the proximal, medial and distal pulsations and the pressure and flow measurements for the three groups are shown in Tables1, 2 and 3. The pressure and flow measurements represent the means of the maximum values of pulsation for each protocol and the resistance is the division of pressure by flow.
Control group measurements.
| Measurement | A | K | KMCh |
|---|---|---|---|
| Pressure (cmH2O) | 71.8±1.8 | 72.5±1.7 | 73.4±1.5 |
| Flow (mL/s) | 13.1±0.2 | 13.0±0.1 | 13.0±0.2 |
| Resistance (cmH2O/mL·s-1) | 5.5±0.1 | 5.6±0.2 | 5.7±0.2 |
| Pulsation – distal (%) | 5.1%±2.1% | 5.4%±2.1% | 5.7%±1.8% |
| Pulsation – medial (%) | 1.5%±1.1% | 2.3%±0.8% | 2.4%±1.0% |
| Pulsation – proximal (%) | 0.6%±0.2% | 1.4%±0.6% | 1.9%±0.9% |
DECEL group measurements.
| Measurement | A | K | KMCh |
|---|---|---|---|
| Pressure (cmH2O) | 62.6±3.3 | 64.0±3.1 | 60.7±3.8 |
| Flow (mL/s) | 13.1±1.1 | 13.0±0.5 | 12.7±1.0 |
| Resistance (cmH2O/mL·s-1) | 4.9±0.3 | 5.0±0.2 | 4.9±0.3 |
| Pulsation – distal (%) | 4.2%±1.7% | 4.3%±1.8% | 4.4%±1.7% |
| Pulsation – medial (%) | 3.2%±1.6% | 2.9%±1.4% | 2.9%±1.4% |
| Pulsation – proximal (%) | 2.7%±2.0% | 2.7%±1.9% | 2.8%±2.2% |
RECEL group measurements.
| Measurement | A | K | KMCh |
|---|---|---|---|
| Pressure (cmH2O) | 61.2±1.8 | 61.0±2.0 | 61.9±2.2 |
| Flow (mL/s) | 14.3±1.2 | 12.9±0.3 | 13.3±0.5 |
| Resistance (cmH2O/mL·s-1) | 4.38±0.3 | 4.73±0.1 | 4.70±0.2 |
| Pulsation – distal (%) | 9.6%±7.9% | 12.5%±9.2% | 9.3%±7.0% |
| Pulsation – medial (%) | 8.0%±3.7% | 8.2%±3.4% | 8.3%±3.6% |
| Pulsation – proximal (%) | 5.6%±2.6% | 5.7%±2.6% | 5.8%±2.6% |
The two-way ANOVA results demonstrated statistically significant variations in the measurements of the trachea in the medial region (between groups; p<0.01) and proximal region (between groups and protocols; p<0.01).
DISCUSSIONTo analyze the mechanical properties of neo-tracheas developed by tissue engineering, an imaging system was created. This system synchronizes the image capture of a camera with the signals obtained by transducers (7). Through the processing and analysis of videos obtained by the camera, it was possible to make quantitative measurements of the variation of tracheal dimensions. To the best of our knowledge, this is the first time that this approach has been used to assess the mechanics of engineered tracheal grafts.
Statistical tests showed significant variations in the measurements of the medial segment (between groups) and proximal segment (between groups and protocols), suggesting that this image capture technique can provide meaningful and quantitative information in addition to the data provided by pressure and flow transducers. The results presented are from a preliminary study. Further studies with more strict methods utilizing the same animal breed, similar assessment times between the groups and standardized tracheal lengths to prevent bias are underway. We also intend to find another external factor that causes more significant changes in the tracheal dimensions to test the potential of this new technique.
In conclusion, the environment used in this study for the assessment of tissue-engineered tracheas is a novel, noninvasive approach that will enable evaluation of the viscoelastic properties of the grafts prior to airway transplantation.
ACKNOWLEDGMENTSThis study was supported by CNPq (National Counsel of Technological and Scientific Development).
AUTHOR CONTRIBUTIONSSilva TH designed and constructed the environment for the measurement of airway respiratory mechanics and participated in the data analysis and manuscript writing. Aoki FG and Valenga MH participated in the data collection, data analysis and manuscript writing. Pazetti R performed the experiments and revised the manuscript. Cardoso PF designed and supervised the experiments and revised the manuscript. Deffune E and Evaristo T produced the tissue-engineered airway segments and revised the manuscript. Pêgo-Fernandes PM revised the manuscript. Moriya HT designed the environment for the measurement of airway respiratory mechanics, supervised the experiments and data analysis and revised the manuscript.
No potential conflict of interest was reported.