This study sought to study the associations of noise with heart rate, blood pressure, and perceived psychological and physiological responses among post-cardiac surgery patients in ICUs.
METHODS:Forty patients participated in this study after recovering from anesthesia. A sound-level meter was placed at bedsides to measure noise level for 42 hours, and patients' heart rate and blood pressure were recorded every 5 minutes. Patients were also interviewed for their perceived psychological/physiological responses.
RESULTS:The average noise level was between 59.0 and 60.8 dB(A) at the study site. Annoyance and insomnia were the respective psychological and physiological responses reported most often among the patients. Although noise level, irrespective of measures, was not observed to be significantly associated with the self-assessed psychological and physiological responses, it was significantly associated with both heart rate and blood pressure.
CONCLUSIONS:Our study demonstrated that the noise in ICUs may adversely affect the heart rate and blood pressure of patients, which warrants the attention of hospital administrators and health care workers.
Hospitals are, supposedly, a place that provides patients with a safe and quiet environment for treatment and recovery. However, hearing contamination in intensive care units (ICUs) as a result of the continually growing use of medical instruments and equipment was found to have an impact on patients' sleep and recovery.1,2 Studies of ICU noise have shown that the mean 24-hour noise level in ICU ranged from 55-65 dB(A), with a maximum level of 80-90 dB(A),3,4 apparently exceeding the noise tolerance level for hospitalized patients. The acceptable sound levels suggested by the U.S. Environmental Protection Agency (EPA)5 are around 40-45 dB(A) for daytime and 35 dB(A) for nighttime.
Noise refers to a powerful, unorganized, irregular, or unwanted sound. It is an unpleasant and hazardous sound that may affect work performance and cause physiological and psychological stress.6 It has a tremendous impact on physiological responses, including tachycardia, hypertension, dyspnea, anorexia, insomnia, thyroxin, and adrenaline increases; delayed wound healing; increased complications; and prolonged hospitalization.7,8 Noise may also pose psychological stress and symptoms such as annoyance, impatience, rage, discontent, excitement, frustration, and uneasiness.7-9 Such unfavorable physiological and psychological effects are expected to disturb the recovery of ICU patients.
Cardiovascular disease is the leading cause of mortality in most developed countries. Cardiac surgery has become a standard treatment for some patients with heart disease. The ICU is a place that, supposedly, provides post-operative care for patients receiving cardiac surgery. To closely monitor patients' conditions, various instruments and monitoring devices are attached to the patients. Unfortunately, the noise generated by these instruments and devices becomes a potential stressor to patients and might, therefore, result in adverse influences on patients' recovery.10,11 Additionally, limited information is available concerning the noise level in ICUs and its impact on patients' heartbeats and blood pressure.12,13 This study sought to measure the noise level in ICUs and to investigate the influences of noise on post-cardiac surgery patients' heart rate, blood pressure, and perceived physiological and psychological responses.
METHODSSubjectsThe subjects purposively sampled in this study were patients treated at cardiac surgical units of a tertiary medical center in Northern Taiwan. Participants were required to be aged 18-75 years with clear consciousness, have experienced first-time cardiac surgery in the absence of surgical complications, be able to communicate verbally without visual or auditory defects, and have their pain well controlled. The exclusion criteria included use of mechanical circulatory support (e.g. intraaotic balloon pumping or extracorporeal membrane oxygenation), use of respirator of FiO2 ≥ 50%, having inotrope equivalent ≥10, arrhythmia requiring anti-arrhythmia drugs, psychiatric illness or cognitive impairment, hemorrhage (chest tube drainage ≥ 100 ml/hr), liver dysfunction (AST ≥ 100 U/L or ALT ≥ 100 U/L, or total bilirubin > 3 mg/dL), kidney dysfunction (BUN > 50 mg/dL, creatinine ≥ 3 mg/dL, or dialysis), and use of tranquilizers.
InstrumentsA sound-level meter (SLM) (NL-22 Type II, RION, Tokyo, Japan) was used to measure noise level in dB(A). The measuring range of the SLM is 20-130 dB(A), with a precision of ± 1.0 dB(A).
In consideration of the ill-health and frailty of cardiac patients who were recovering from surgery, most of the existing questionnaires designed to assess psychological and physiological responses are considered too complex and time-consuming to be administered to these vulnerable patients. Thus, the authors developed the Impact of Noise Perception (INP) questionnaire, which is simple and straightforward, to assess psychological and physiological responses to ICU noise in cardiac surgical patients. The INP comprises 7 items for the assessment of psychological responses (annoyance, startle, anxiety, anger, nervousness, fear, and depression) and 9 items to assess physiological responses (insomnia, tachycardia, easy fatigue, breath distress, dizziness, headache, muscle stress, anorexia, and hearing loss). The study patients were asked to indicate the perceived impact associated with each of the above psychological and physiological items with anchors of 1 (not affected at all) to 5 (severely affected). Prior to its implementation in the study, the INP was assessed for its validity, and some revisions were made accordingly to improve the validity and reliability of the INP. Six experts with expertise in nursing and surgery were invited to verify the necessity and accuracy of each item. The internal consistency reliability coefficient (Cronbach's α) of the INP based on the data of this study was estimated at .84 and .85 for the psychological and physiological responses, respectively.
The vital parameters, including heart rate (HR), systolic arterial blood pressure (SABP), diastolic blood pressure (DABP), and mean arterial blood pressure (MABP), were measured and recorded using a physiologic monitor (Hewlett-Packard M1166A, Model 66S, USA). Physiologic monitors are tested and calibrated annually by bioengineering departments.
ProceduresAfter being comprehensively informed, all study participants gave informed consent on the day prior to surgery. Audiometer screening was assessed on the same day to exclude those who had a hearing impairment.
The study participants were prescribed morphine HCL (10 mg/ml) PRN Q4H/IV 1A soon after surgery. Additional prescriptions were administered thereafter every 4 hours if necessary. Two hours after completion of surgery, all study participants had recovered from anesthesia and regained full consciousness. The collection of noise level experienced by each patient and measurements of patients' vital parameters started around 17:00-20:00 after making sure that subjects were free of complications. The SLM was placed at the bedside some 30 cm away from patients' heads to measure the noise level every second continuously for 42 hours. The noise at the study sites was, generally, caused by devices (such as ventilators, infusion pumps, and monitors) that are used during the delivery of care, often to support or optimize the patient's physiological status. Additionally, medical bleeps, telephones ringing, and alarms triggering clinical devices can also be a source of noise in the study ICUs, and the frequency of ICUs was, essentially, constant during the patients' stay in ICUs. Patients' HR and BP were measured every 5 minutes during the same time period within which the noise was measured. Two investigators (SMH and SLH) operated and monitored the collection of noise level and patients' vital parameters.
Patients' psychological and physiological responses were also assessed on the day of discharge from the study ICU. Face-to-face interviews were conducted to obtain information on psychological and physiological responses, and all interviews were carried out by the principal investigator (SMH) to avoid biased information. The study protocol was reviewed and approved by the Institutional Review Board of the collaborative hospital.
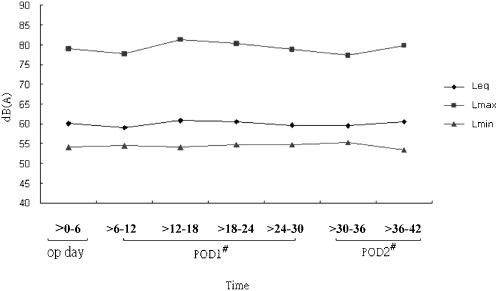
Statistical analysisData were analyzed using SPSS for Windows 12.0. Descriptive statistics were used to characterize the patients' demographic characteristics, exposure to noise level, and psychological/physiological responses. Spearman's rank order correlation was used to assess the relationships between noise level and psychological and physiological responses. Over the 42-hr period, the mean (Leq) noise level was calculated as the average of the hourly mean noise levels, while the maximum (Lmax) and minimum (Lmin) are the average of the hourly maximum and minimum noise level, respectively.
Noise level was, further, calculated separately for seven 6-hr time periods following patients' recovery. The Leq, Lmax, and Lmin of noise levels were calculated for each time period. Cross-sectional time-series regression14 was used to examine the relationships between vital parameters and noise levels. Since nine cases were discharged from the study ICU less than 42 hours after noise measurement began, they were excluded from this regression analysis.
RESULTSForty-one subjects were eligible to participate in this study, but one was later excluded due to delirium. A total of 40 participants completed the study. The majority of them were male, with a mean age of 54.5 ± 14.5 years. Most of the study subjects were married, employed, and without prior ICU experience. One-third of the subjects underwent coronary artery bypass graft surgery, and the others had valvular surgeries (Table 1).
Characteristics of the study participants (n = 40).
| Variables | N | % |
|---|---|---|
| Gender | ||
| Male | 35 | 87.5 |
| Female | 5 | 12.5 |
| Age (years) | ||
| <40 | 8 | 20.0 |
| 41-50 | 7 | 17.5 |
| 51-60 | 10 | 25.0 |
| >60 | 15 | 37.5 |
| 54.5±14.5 | ||
| Marital Status | ||
| Married | 33 | 82.5 |
| Single | 7 | 17.5 |
| Education | ||
| Middle school or less | 17 | 42.5 |
| High school | 9 | 22.5 |
| College/university/graduate | 14 | 35.0 |
| Employment | ||
| Yes | 25 | 62.5 |
| No | 15 | 37.5 |
| Religion | ||
| Yes | 28 | 70.0 |
| No | 12 | 30.0 |
| Operation | ||
| CABG | 25 | 62.5 |
| Valvular surgery | 15 | 37.5 |
| Previous ICU experience | ||
| Yes | 13 | 32.5 |
| No | 27 | 67.5 |
CABG: Coronary artery bypass grafting, ICU: intensive care unit
Data on noise level were divided into seven 6-hr time periods (Figure 1). The average hourly noise level (Leq) calculated for each time period ranged from 59.0 to 60.8 dB(A); the average hourly maximum (Lmax) and minimum (Lmin) noise level was in the range of 77.3-81.3 dB(A) and 53.4-55.3 dB(A), respectively.
Patients in ICUs perceived minor psychological responses to noise. The top 3 psychological responses were annoyance, startle, and anxiety. However, the measured noise level was not significantly associated with any of the psychological responses (Table 2). The top 3 physiological responses to noise were insomnia, tachycardia, and easy fatigue. Again, the noise level determined in ICUs was not significantly associated with any of the physiological responses of interest (Table 3).
Scores of psychological responses and their correlations with noise level, indicated by Spearman rho coefficients (n = 40).
| Spearman rho according to measures of noise level | ||||
|---|---|---|---|---|
| Psychological responses | Score∗ | Leq† | Lmax‡ | Lmin§ |
| Annoyance | 1.83±1.22 | 0.20 | 0.27 | 0.08 |
| Startle | 1.63±1.03 | 0.01 | 0.20 | -0.05 |
| Anxiety | 1.58±1.11 | 0.06 | 0.21 | 0.01 |
| Anger | 1.55±1.08 | 0.22 | 0.20 | 0.23 |
| Nervousness | 1.50±0.96 | 0.01 | 0.17 | -0.01 |
| Fear | 1.45±0.81 | 0.08 | 0.15 | 0.03 |
| Depression | 1.30±0.85 | 0.09 | 0.25 | -0.01 |
Scores of physiological responses and their correlations with noise level, indicated by Spearman correlation coefficients (n = 40).
| Spearman rho according to measures of noise level | ||||
|---|---|---|---|---|
| Physiological responses | Score1∗ | Leq† | Lmax‡ | Lmin§ |
| Insomnia | 2.05±1.28 | 0.09 | 0.24 | -0.03 |
| Tachycardia | 1.40±0.71 | -0.21 | -0.12 | -0.23 |
| Easy fatigue | 1.33±0.80 | 0.20 | 0.16 | 0.11 |
| Breath distress | 1.20±0.46 | -0.01 | -0.03 | 0.06 |
| Dizziness | 1.20±0.65 | -0.02 | 0.04 | -0.12 |
| Headache | 1.18±0.59 | -0.07 | 0.17 | -0.18 |
| Muscle stress | 1.18±0.59 | -0.13 | 0.06 | -0.16 |
| Anorexia | 1.05±0.22 | 0.30 | 0.12 | 0.35 |
| Hearing loss | 1.05±0.22 | -0.19 | -0.22 | -0.11 |
The results of the time-series regression on noise levels and heart rate and blood pressure showed that an increase in noise level was positively and significantly associated with heart rate (HR), systolic arterial blood pressure (SABP), diastolic blood pressure (DABP), and mean arterial blood pressure (MABP) (Table 4). A one dB(A) increase in noise level in the ICU was associated with an increase of HR, SABP, DABP, and MABP by 0.07 beats/min, 0.58 mmHg, 0.15 mmHg, and 0.53 mmHg on average, respectively (Table 4). The standardized beta tended to indicate that noise may have greater influences on SABP (β = 0.26) and MABP (β = 0.24) than on the other vital parameters.
DISCUSSIONA quiet environment is essential for patients to recover from illness. Therefore, noise level control is crucial in hospitals, especially in ICUs. Cardiac surgical patients usually encounter tremendous physical and psychological threats from surgery. To maintain and monitor their vitals, they are attached to many different instruments and devices, such as a respirator, electrocardiograph, blood pressure meter, central venous pressure meter, pulmonary artery catheter, and so on. Noise generated by these instruments and devices becomes a potential stressor and brings negative effects to patients. This study found that the mean sound level in the study ICU was in the range of 59.0-60.8 dB(A). Although this sound level was similar to3,15-17 or even lower18,19 than the findings reported by some previous studies, it is much higher than the regulatory sound level [40-45 dB(A) and 35 dB(A) during daytime and nighttime, respectively].5 Additionally, the sound level was fairly consistent in different time periods over the study period, indicating that cardiac patients tended to be constantly exposed to a noisy environment. Because medical technology advances with time, there has been an increasing amount of equipment and instruments used in ICUs, so clinicians and hospital administrators should consider control strategies that can effectively reduce patients' exposure to such a high noise level to ensure a quiet environment for cardiac patients to rest and recover, especially during the late night period.
Noise is an important factor in making people feel discomfort. Annoyance, startle, and anxiety were the top three psychological responses observed in our sample patients, which is similar to the findings of some other previous studies that found that the patients in ICUs were likely to be irritated and disturbed with impatient, distressed, anxious, or angry emotions.20-22 On the other hand, insomnia, tachycardia, and easy fatigue were the top three perceived physiological responses in our patients, which is also similar to those reported in previous studies.23,24 Despite that, the potential influence of noise on patients' psychological and physiological responses found in our patients seemed minor (between not affected at all to mildly affected). Our study found little association between noise level and psychological and physiological responses. Perceived psychological and physiological responses to noise are often complicated and can be affected by an individual's age, culture, social factors, and personal susceptibility. The interpretation of and feeling about noise differ with the type of sound, past experience with the same sound, and individual adaptation.25-27 Patients in Taiwan might recognize that noise is, surely, accompanied by treatment procedures; hence, it is acceptable and unavoidable. This could be responsible for the little association between noise level and psychological and physiological responses of the patients. Furthermore, our study was limited in noise level measurement to only 2 days, and the information concerning the potential influences of noise exposure for a longer period of time was not available in our study.
Noise may stimulate the hypothalamus-pituitary-adrenal gland system to trigger a series of stress hormones that affect tissue synthesis and metabolism in human bodies.7,28,29 Physiological effects from exposure to noise include increased peripheral vascular resistance, heart rate, and blood pressure leading to enforced ventricular contractility, hypertension, and subsequent cardiac hypertrophy; hence, it increases the risk of ischemic heart disease.12,13,30,31 To our knowledge, our study is the first to monitor and examine the effects of noise on heart rate and blood pressure continuously for as much as 42 hours. A number of previous studies were conducted to examine the physiological effects of noise on patients. The findings of those studies are, however, neither consistent nor convincing. Garvin et al.32 investigated the cardiovascular responses of cardiac ICU patients when communicating with nurses, physicians, and families and found that noise may significantly increase maximum heart rate, while the influence of noise on minimum heart rate or BP (diastolic and systolic) was not statistically significant. Baker12 investigated the effect of noise on the heart rate of 28 surgical ICU patients. The noise in ICUs and the heart rate of patients were measured continuously for 6 hours. The results showed that the noise increased heart rate in 68% patients and decreased it in 11%. In another study, Baker13 selected 20 cardiac ICU patients to further investigate the effect of noise on heart rate and blood pressure and found that the maximum heart rate was positively and significantly associated with noise level, while no significant associations between noise and minimum heart rate or blood pressure were noted. Our study demonstrated that the vital parameters including HR, SABP, DABP, and MABP were all positively and significantly associated with an increase in ICUs noise levels. Comparisons between our study findings and those previously reported might be difficult mainly because the potential physiological influence of noise might be affected by the disease and demographic characteristics of patients and by factors related to health care.
Although we did not record the load of morphine received by each of the study participants, the potential influence of sedative load on patients' perception is unlikely to confound the relationships between noise level and vital parameters because the dosage of morphine prescribed for the study participants should be unrelated to the noise level experienced by the study participants. Additionally, because all of the study participants were at full consciousness and free of complications at the time of interview, failure to adjust for sedatives load would not have a notable influence on our findings.
Our study showed that annoyance and insomnia are the major psychological and physiological responses in cardiac patients in ICUs. Although noise level was not significantly associated with the perceived psychological and physiological responses of ICU patients, our study did demonstrate that the noise generated by ICU medical devices was positively and significantly associated with increased heart rate and blood pressure, which warrants attention from health care workers and hospital administrators, who should consider a strategy that can effectively control the noise level in ICUs.