To analyze the effects of motor intervention on the neuropsychiatric symptoms of Alzheimer's disease and on the caregivers' burden.
DESIGN:This is a controlled trial evaluating the effects of a motor intervention program on the neuropsychiatric symptoms.
SETTING:The intervention was performed on community patients from two university centers specializing in physical exercise for the elderly.
SUBJECTS:Patients with Alzheimer's disease were divided into two groups: sixteen received the motor intervention and sixteen controls (five controls were excluded because of clinical intercurrences).
INTERVENTIONS:Aerobic exercises (flexibility, strength, and agility) and functional balance exercises were conducted over six months for 60 minutes three times per week.
MAIN MEASURES:Psychopathological features of patients were evaluated with the Neuropsychiatric Inventory and Cornell Scale for Depression in Dementia. Caregivers were evaluated using the Neuropsychiatric Inventory-Distress and Burden Interview. A two-way analysis of variance (ANOVA) was applied to observe interactions (pre- vs. post-intervention; participants vs. controls).
RESULTS:Patients from the intervention presented a significant reduction in neuropsychiatric conditions when compared to controls (Neuropsychiatric Inventory: F:11.12; p = 0.01; Cornell Depression scale: F:11.97; p = 0.01). The burden and stress of caregivers responsible for patients who participated in the intervention significantly decreased when compared to caregivers responsible for controls (Neuropsychiatric Inventory-Distress: F: 9.37; p = 0.01; Burden Interview: F: 11.28; p = 0.01).
CONCLUSIONS:Aerobic exercise was associated with a reduction in the neuropsychiatric symptoms and contributed to attenuate the caregivers' burden. However, the researchers were not blinded to the patient's intervention status, which constitutes an important limitation of this study.
Neuropsychiatric symptoms are important clinical manifestations of Alzheimer's disease (AD), which is a neurodegenerative disorder that causes a decrease in cognition, functionality, and behavior.1,2 Although the major etiological agent is the neurodegenerative process causing the dementia, there is a complex interplay between the biological, social, and psychological factors that are involved in the pathogenesis of these symptoms.3 General clinical conditions, environmental stressors, and demand surpassing the caregiver's capacity may result in psychopathological manifestations.1 These symptoms are highly prevalent and represent a source of burden for the caregiver, resulting in a shorter time to institutionalization4 and an increase in the suffering of the caregivers or relatives.5 In this context, caregivers of AD patients display important burdens associated with their daily caregiving responsibilities because of the frequency of and the stress related to the provision of assistance.6
At present, pharmacological interventions for AD patients with behavioral disturbances are limited to the treatment of symptoms, although treated patients have shown significant benefits compared to those without treatment.7 Improving the management of AD patients with neuropsychiatric symptoms such as agitation, aggression, depression, apathy, and other psychopathological phenomena requires the attenuation of the frequency and severity of these disturbances.
Whereas studies have increasingly targeted the retardation of cognitive decline through pharmacological or non-pharmacological strategies, few studies have focused on the use of aerobic exercise to alleviate neuropsychiatric symptoms and reduce the caregiver's burden in AD. Research concerning the effects of exercise on the risk of dementia in the elderly has produced inconsistent results, with some studies, but not others,10,11 reporting a lower risk of all-cause dementia, including AD.8,9 However, in addition to traditional medical approaches to prevent disease progression, regular exercise may significantly improve well-being and reduce the risk of dementia later in life.9 Consequently, there is currently a significant, increasing effort to clarify the impact of physical exercises on the risk of dementia in this neurodegenerative condition. Recently, Hamer & Chida12 used a meta-analysis to conclude that physical exercise is protective against the future risk of dementia and reduces the risk of AD.
Aiming to reduce disability in patients with AD, Teri et al.13 performed a randomized controlled trial that demonstrated the favorable effects of exercise on behavioral management and on depressive symptoms among patients with AD in comparison to those under routine medical care. Landi et al.14 similarly reported that aerobic exercise reduced neuropsychiatric symptoms, such as wandering, aggression, and sleep disturbances, in nursing home residents who were cognitively impaired. In this same context, Heyn et al.15 demonstrated by a meta-analysis that regular and systematic aerobic exercise, such as walking, dancing, and cycling, provided favorable effects on dementia, including AD. The authors attributed these benefits to a reduction in cognitive decrease and to an improvement in daily living activities.
However, in another meta-analysis, Forbes et al.16 found no similar benefits of global physical activity programs for persons with dementia. After a one-year exercise program, Rolland et al.17 similarly failed to observe a significant decrease in the behavioral symptoms of AD.
There is emerging but insufficient evidence that exercise prescription could be an established treatment for neuropsychiatric symptoms in AD.18 Furthermore, more scientific support is needed to clarify whether exercises targeted at patients can reduce the caregiver's burden and the suffering of relatives. To address this question, we hypothesized that an appropriate motor intervention program could contribute to a decrease in the psychopathological disturbances of AD and attenuate the caregiver's burden.
The purpose of this study was to examine the effects of a six-month aerobic exercise program on the neuropsychiatric symptoms of patients with AD and the impact of exercise on alleviating the caregiver's burden.
METHODSThis controlled trial involved 32 community patients and their respective caregivers recruited from two centers: the Aging and Physical Activity Laboratory (UNESP – Univ Estadual Paulista) and the Alzheimer's Brazilian Association (Regional-Araras, SP).
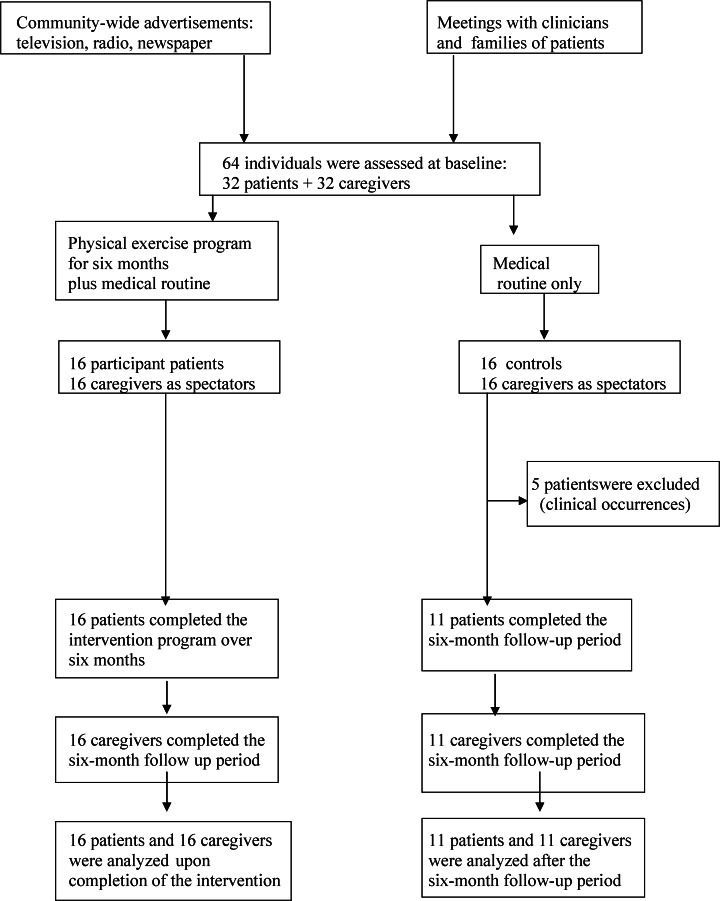
Several strategies were implemented for the recruitment of subjects to the physical exercise program: a) explanations to clinicians about the importance of our research project; b) community-wide advertisements on television, newspaper and radio; and c) community meetings to present the objectives and development stages of our project. After these disclosures, families and clinicians began to refer their patients to our services. The physical exercise program for AD was implemented at UNESP – Univ Estadual Paulista in 2003, and the patients from the present study were assessed at the beginning of 2008.
Inclusion criteria were as follows: (a) diagnosis of probable AD by a trained clinician; (b) mild or moderate stage of the disease; (c) locomotion ability, preserved vision and hearing (eyeglasses and/or hearing aid were permissible) sufficient for compliance with testing procedures; and (d) presence of a respective caregiver. Patients with motor disorders who had difficulty with the motor performance required for physical tasks were excluded.
The clinicians responsible for the patients established the diagnosis of Alzheimer's disease according to the consensus criteria of the National Institute for Neurological and Communicative Disorders/Association (NINCDS/ADRDA).19 They referred the patients to our services for participation in the present investigation. The assessment of dementia was made according to the Diagnostic and Statistical Manual of Mental Disorders.20 The dementia severity was measured according to the Clinical Dementia Rating scale.21 This scale assesses the patient's cognitive and functional state, and it is graded on a scale from 0 (normal) to 0.5 (questionable dementia), 1 (mild dementia), 2 (moderate dementia), and 3 (severe dementia). A physician trained in geriatric psychiatry confirmed the diagnosis to include only patients with mild or moderate AD. Furthermore, he supervised all cognitive and neuropsychiatric assessments while blind to the patient's assignment to the motor intervention or control groups.
The main outcome measures concerned the impact of aerobic exercise on neuropsychiatric symptoms in AD. To this end, the interviewers completed a cognitive assessment at each time point (baseline and final application) using the Mini-Mental State Examination22 to characterize global cognitive functioning. This instrument is a brief, cognitive screen that assesses the patient's time and place orientation, immediate and delayed recall, concentrated attention and calculation, naming, repetition, comprehension, and constructional praxis. The interviewers additionally applied the Neuropsychiatric Inventory23 to identify psychopathological features including delusions, hallucinations, agitation, dysphoria, anxiety, euphoria, apathy, dis-inhibition, irritability, aberrant motor behavior, sleep disorders, and appetite disorders. This instrument consists of a structural interview for measuring the frequency and severity of symptoms, with higher scores related to greater severity. Moreover, the Neuropsychiatric Inventory allows the assessment of the caregiver's distress related to patient disturbances. To determine the caregiver's mental suffering and burden, the interviewers completed the Burden Interview,24 and to identify depressive symptoms in the patients, they applied the Cornell Scale for Depression in Dementia,25 with higher scores representing greater severity. Two experts on neuropsychiatric disorders in the elderly supervised all cognitive and psychopathological procedures.
The interviewers administered the scales to patients (e.g., the Mini-Mental State Examination) and caregivers (e.g., the Neuropsychiatric Inventory-Distress and Burden Interview) either together or separately when appropriate, taking into account the patient's behavior or emotional condition. In general, the interviewers questioned the caregiver while observing the patient when they were together, and the interviewers requested the patient's attention for clarifying some items when necessary. In conducting these procedures, the interviewers made an effort to limit the emotional influence of the patient or the caregiver on the results. At baseline, the raters were unaware as to how the groups would be divided. In addition, the raters themselves did not know the group to which the patient and the caregiver belonged.
Although the raters had planned to be blinded in completing the scales, this strategy was not implemented because of the insufficient number of experts.
At baseline, prior to the start of the study, no patients had been engaged in a systematic physical exercise program. The patients were divided into two groups to generate groups with overall similar physical activity levels, as measured by the Modified Baecke Questionnaire for the Elderly,26 and similar psychopathological conditions, as measured by the Neuropsychiatric Inventory (total score) and the Cornell Scale for Depression in Dementia. Following group assignment, the 16 patients in the treatment group participated in the exercise program plus a medical routine, whereas the 16 patients in the control group abstained from systematic physical exercise and adhered to their standard medical routine (based on prescribed medicines and general health orientation established by their respective clinicians, excluding participation in physical exercise programs). Neither the patients nor the caregivers were informed of their allocation to one group or the other prior to the start of the trial.
For the treatment group, exercises were performed over a six-month period for 60 minutes three times per week on nonconsecutive days. Exercises were conducted in locations and buildings with appropriate conditions that were, in general, close to the homes of the participants. Patients came to the sites with their caregivers by automobile or by special public transport, and this travel did not involve systematic physical exercise. Patients in the control group traveled to the exercise sites only on the days of the assessment: once at the beginning and once at the end of the six-month period. During the intervention, patients from the control group stayed at home or performed the typical activities of daily life. Researchers ensured that these patients refrained from systematic motor intervention during this period and instead followed only their regular medical routine. In addition, after the end of the exercise program, the patients from the control group were invited to engage in other physical activity studies performed at the same sites.
The aerobic exercise included five phases suggested by Gobbi et al.27 for the elderly in Brazil. Each exercise session was comprised of a warm-up period, pre-exercise stretching, the main exercise procedures, a cool-down period, and post-exercise stretching. At the end of each phase, there was a progressive increase of load. The main exercise program involved aerobic procedures such as walking, dancing, and exploring upper and lower limb mobility with the goal of enhancing flexibility, strength, and agility as well as improving functional balance. There is evidence to suggest that these motor components are important for maintaining the functional capacity of patients with AD.
Sixteen patients participated in the program of aerobic exercise with moderate intensity and on a regular basis over six months. The aerobic exercises (moderate intensity over a long duration) encompassed activities that simultaneously benefit several components of functional capacity such as flexibility (stretching), muscular resistance (specific exercises for the large muscle groups), motor coordination (rhythmic activities), and balance (recreational motor activities). The exercise program was administered to two subgroups of eight participants simultaneously, each under the supervision of at least one expert on physical education.
The respective caregivers did not participate in this program. They observed the activities of their patients (exercise program plus medical routine vs. medical routine only) during the six-month period from a distance as spectators. In addition, the caregivers did not engage in the interventions themselves. This strategy aimed to assess effects on the caregivers' burden that could be attributed to the hypothesized reduction in neuropsychiatric symptoms of the respective participant patients. The caregivers were evaluated with respect to their burden and the occurrence of stress at the beginning and the end of this period using the Neuropsychiatric Inventory-Distress23 and Burden Interview questionnaires.24
Data processing and statistical analysis were performed using the ‘Statistica 5.0' software. The descriptive analyses displayed mean values and standard deviations. The Shapiro-Wilk test was performed to assess the normal distribution of the data, and because the confirmed data were normal, a parametric analysis was considered. First, the t-test was applied to independent samples to verify group homogeneity for baseline values. The demographic and clinical variables of the groups were compared using the two-way analysis of variance (ANOVA) for repeated variables. The two-way ANOVA was similarly applied to verify interactions between time points (pre- and post-intervention) and groups (with motor intervention and controls). Because five patients left the motor intervention group before the completion of the trial, a complete case analysis was made. Statistical significance was defined at a level of p<0.05.
The local ethics committee approved the study, and patients and/or relatives (directly responsible for patients) provided written informed consent.
RESULTSThe sample comprised 32 community patients with AD and their respective caregivers. At baseline, 34% of all patients were receiving anticholinesterases, 17% were receiving antidepressants, and 12% were receiving benzodiazepines. The severity of dementia was rated from 1 (mild) to 2 (moderate) according to the Clinical Dementia Rating scale.21 The socio-demographic characteristics of the patients, together with selected clinical aspects, are displayed in Table 1.
Socio-demographic and clinical aspects of 32 patients with Alzheimer's disease and their respective caregivers at baseline (percentage, mean and standard deviation).
| Patients (n = 32) | Caregivers (n = 32) | |
|---|---|---|
| Characteristics | (%, mean and SD) | (%, mean and SD) |
| Gender | ||
| - Male | 12 (37.5%) | 2 (6.3%) |
| - Female | 20 (62.5%) | 30 (93.7%) |
| Age (years) | 77.8 (5.8) | 54.2 (11.7) |
| Mini-Mental (points) | 15.4 (6.0) | ----- |
| Education (years) | 4.5 (4.1) | 9.8 (4) |
| CDR (points)Diagnosis duration (years) | 1.52.8 (1.3) | ---------- |
| Care duration (years) | ----- | 2.7 (1.4) |
CDR: Clinical Dementia Rating; Mini-Mental: Mini-Mental State Examination.
SD: Standard deviation.
During the exercise program, five patients from the control group suffered severe clinical occurrences such as stroke or falls with fractures and sudden cognitive decline. Because of these events, these patients and their respective caregivers were excluded from the sample. This group was composed of 11 patients and 11 caregivers at the end of the study (Figure 1). The five patients who were lost from the control group received appropriate medical care.
Participants from the exercise group attended at least 70% of the motor sessions.
At baseline, both groups had similar levels of psychopathological manifestations, as measured by their Neuropsychiatric Inventory scores (participants vs. controls: 40.3 vs. 39.6; t-test: -0.4; p = 0.69), which suggests these manifestations were clinically relevant. However, after the six-month period of the aerobic program, the Neuropsychiatric Inventory scores of the participants had decreased and were lower than those of the controls (40.3 to 16.9 vs. 39.6 to 43.3) with significant differences between the groups (two-way ANOVA: F:11.12; p = 0.01). Similarly, when examining the Cornell Scale for Depression in Dementia, the participants' scores decreased over the six-month period, and they presented significantly lower values than the controls (11.8 to 6.4 vs. 12 to 17.1; t-test: 0.25; p = 0.81), with significant differences between groups (two-way ANOVA: F:11.97; p = 0.01). Here, the main differences were based on a reduction in neuropsychiatric symptoms among participants (agitation/aggression, depression, anxiety, apathy/indifference, dis-inhibition, irritability, and appetite alterations) and an increase in these symptoms among patients in the control group (Table 2; Figure 2).
Neuropsychiatric symptoms according to Neuropsychiatric Inventory domains and the Cornell Scale for Depression in Dementia for patients with Alzheimer's disease (significant values: mean and SD) at baseline and at the time of final evaluation, after six months of exercise.
| Controls(n = 11) | Patients with motor intervention (n = 16) | |||||
|---|---|---|---|---|---|---|
| Neuropsychiatry Inventory | First evaluation | Final evaluation | First evaluation | Final evaluation | F | p |
| (mean, SD) | (mean, SD) | (mean, SD) | (mean, SD) | |||
| Delusions | 4.3 (4.7) | 2.5 (3.2) | 1.9 (3.7) | 0.4 (1.1) | 0.01 | 0.90 |
| Hallucinations | 3.7 (5) | 1.7 (3.6) | 1.4 (3.1) | 0.1 (0.3) | 0.11 | 0.73 |
| Agitation/aggression | 2.7 (3.9) | 5 (5.4) | 2.4 (3.3) | 0.3 (1) | 5.52 | 0.02 |
| Depression | 4.5 (4.1) | 6.2 (5) | 5.3 (5.1) | 1.4 (3.1) | 6.4 | 0.01 |
| Anxiety | 5.4 (4.9) | 6.8 (5.7) | 6.6 (4.7) | 1.3 (3.2) | 9.14 | 0.01 |
| Euphoria | 2.4 (4.8) | 0.2 (0.6) | 1.5 (4.1) | 0 | 0.15 | 0.69 |
| Apathy/indifference | 4.5 (4.5) | 6 (4.6) | 6.4 (5.2) | 1.4 (3.2) | 8.87 | 0.01 |
| Dis-inhibition | 0.5 (1.2) | 1.4 (2.8) | 2.7 (4.5) | 0.1 (0.3) | 4.95 | 0.03 |
| Irritability | 5.6 (5.4) | 6.9 (5.3) | 6.6 (4.4) | 0.8 (3) | 10.95 | 0.01 |
| Motor behaviors | 2.5 (4) | 1.1 (2.4) | 2.9 (4.6) | 0.5 (1.4) | 0.29 | 0.59 |
| Sleep disturbances | 4.4 (5.6) | 2.7 (4.7) | 2.8 (4.2) | 0.3 (0.7) | 0.27 | 0.60 |
| Appetite alterations | 1.1 (2) | 2 (2.5) | 3.6 (4.3) | 0.9 (3) | 8.03 | 0.01 |
| NPI-Total | 39.6 (25) | 43.3 (18.4) | 40.3 (34) | 16.9 (17.6) | 11.12 | 0.01 |
| Cornell Depression | 12 (7.9) | 17.1 (6.3) | 11.8 (6.5) | 6.4 (4.8) | 11.97 | 0.01 |
Values of F interaction between group and motor intervention and ANOVA two-way for repeated measures.
SD: Standard deviation.
With regard to the burden and stress of the caregivers, at baseline, the scores on the Neuropsychiatric Inventory-Distress and Burden Interview were high in both groups. However, there were no significant differences between these caregiver groups (Neuropsychiatric Inventory – total burden score: 18.3 vs. 20.1; t-test: -0.24; p = 0.81; Burden Interview: 32.3 vs. 36.4; t-test: -0.57; p = 0.57). These scales measure the burden and stress, respectively, experienced by the caregivers of patients with dementia.1,28 However, caregivers whose patients participated in the aerobic program presented a significant reduction in Neuropsychiatric Inventory-Distress (18.3 to 3.3). Conversely, caregivers whose patients belonged to the control group maintained similar scores at the end of the program (19.6 to 20.4), with a significant difference between groups (two-way ANOVA: F:9.37; p = 0.01). In the Burden Interview, the scores of caregivers whose patients participated in the aerobic program similarly significantly decreased (32.3 to 14.6), whereas the scores of caregivers whose patients belonged to the control group remained the same (35.6 to 35.5). The difference between the groups was significant (two-way ANOVA: F:11.28; p = 0.01) (Table 3).
Caregivers' burden and stress (significant values: mean and SD) related to Neuropsychiatric Inventory domains and Burden Interview among patients with Alzheimer's disease at baseline and at the time of the final evaluation, after six months of exercise.
| Caregivers of Controls (N = 11) | Caregivers of Participants (N = 16) | |||||
|---|---|---|---|---|---|---|
| Neuropsychiatric Inventory-Distress | First Evaluation (mean, SD) | Final Evaluation (mean, SD) | First Evaluation (mean, SD) | Final evaluation(mean, SD) | F | p |
| Agitation/aggression | 1.6 (2) | 2.2 (2.2) | 1.6 (2.1) | 0.1 (0.5) | 4.98 | 0.03 |
| Anxiety | 2.5 (2) | 2.8 (2.4) | 2.5 (1.9) | 0.4 (1) | 8.83 | 0.01 |
| Apathy/indifference | 2.2 (1.9) | 2.8 (1.9) | 2.8 (2) | 0.6 (1.3) | 10.23 | 0.01 |
| Dis-inhibition | 0.4 (0.8) | 0.9 (1.6) | 1 (1.7) | 0.1 (0.3) | 5.00 | 0.03 |
| Appetite alterations | 0.8 (1.3) | 1.5 (2) | 1.6 (1.9) | 0.4 (1.2) | 6.74 | 0.01 |
| NPI-Distress (total) | 19.6 (14.3) | 20.4 (9.6) | 18.3 (13.8) | 3.3 (6.5) | 9.37 | 0.01 |
| Burden Interview | 35.6 (14.9) | 35.5 (13.7) | 32.3 (14.7) | 14.6 (8.1) | 11.28 | 0.01 |
Values of F interaction between group and motor intervention and ANOVA two-way for repeated measures.
SD: Standard deviation.
The improvement in the caregivers' burden was attributed to a reduction in the behavioral disturbances by patients who performed the exercise program. For instance, agitation/aggression, anxiety, apathy/indifference, dis-inhibition, and appetite alterations, as well as depression, were reduced in this group. However, caregivers whose patients belonged to the control group did not experience the same benefit (Figure 3).
DISCUSSIONThe results of this analysis indicate that aerobic exercise over a six-month period contributed to a decrease in the neuropsychiatric symptoms of patients with AD and to a reduction in caregiver burden. In the control group, these symptoms slightly increased, and the burden of the respective caregivers remained unchanged. It should be noted that during the intervention, five patients were excluded from the control group. The primary reasons for exclusion were clinical intercurrences, such as stroke or falls, which were associated with a severe cognitive decrease. Because it is possible that these patients differed from those who experienced no intercurrences, they were excluded to maintain the original features of the control group. For this reason, a complete case analysis was performed.
Patients who participated in aerobic exercise experienced improvement in several domains on the Neuropsychiatric Inventory, including irritability, anxiety, apathy/indifference, and appetite alterations, when compared to controls. Differences between the groups could be attributed to the reciprocal influence of two factors, i.e., the reduction in symptoms among patients who participated in aerobic exercise and the increase of these symptoms in the control group. This interaction was significant but was simultaneously based on a decrease in psychopathological manifestations in the exercise group and an increase of these symptoms in the control group. Therefore, because AD is a neurodegenerative process with progressive impairment of cognition and behavior, aerobic exercise is a useful strategy for alleviating patient suffering and contributed to a reduction in caregiver stress by allowing the patients to better manage their own daily responsibilities.29–31 In addition, apathy/indifference, anxiety, and appetite alterations represented the primary sources of caregiver burden. Unsurprisingly, behavioral disturbances rather than cognitive impairment have been previously reported to be important factors linked to patient and caregiver burden.1,3
Two meta-analyses15,16 that examined the effect of physical activity on cognition, function and behavior in patients with dementia arrived at distinct conclusions. Whereas Heyn et al.15 verified that individuals with cognitive impairment or dementia who took part in an exercise program experienced benefits to cognition, daily life activities, and behavioral disturbances, Forbes et al.16 found no sufficient evidence to support similar conclusions. Additionally, Steinberg et al.32 recently verified the benefits of physical exercise for functionality in basic daily activities, but not for behavioral disturbances. To measure psychopathological symptoms, these authors32 applied the Neuropsychiatric Inventory, which is the same scale used in our investigation. Despite a growing number of studies that emphasize the effects of aerobic exercise on cognitive function in AD, there are few reports in the literature aimed at analyzing the impact of such exercise on behavioral disturbances and functionality.
Our findings are in agreement with studies reporting that physical exercise may be associated with the attenuation of neuropsychiatric symptoms in AD,13,14,17,30,31 but diverge from the conclusions of others.16,32,33
Alternative methodological strategies that could be implemented may produce different results compared with our data. For instance, reductions in neuropsychiatric symptoms may have been improved if the caregivers were performing the same aerobic program as the patients. Furthermore, this type of research is impacted by the difficulty of measuring both the effects of physical exercise itself and the psychosocial stimulation that may influence the patient's motivation. In addition, if the control group had been provided with joint activities, such as recreational games with their caregivers during the same period of time, their neuropsychiatric symptoms may have been improved. These questions remain to be answered.
It should be noted that the Neuropsychiatric Inventory-Clinician rating scale is a uniform assessment system for accurately measuring behavioral disturbances in patients with dementia and caregivers' burden.34 This scale includes a clinician-rating judgment to provide an overall severity rating for neuropsychiatric symptoms in dementia.
In Brazil, Fialho et al.35 recently reported a significant association between the behavioral manifestations of patients with dementia, as assessed by the Neuropsychiatry Inventory, and a high level of burden among caregivers. The similarly favorable scores from both the Neuropsychiatric Inventory-Distress and Burden Interview questionnaires suggests that regular aerobic exercise should help to attenuate the caregivers' suffering. In addition, given the limited efficacy of pharmacological strategies for AD, exercise intervention could represent a useful resource to delay the need for placement of patients under a complex level of care.32
Concerning the neurobiological approach, exercise has been related to enhance neuronal survival36, vascularization, and growth factors in the brain regions linked to cognitive functions such as memory.37 In experimental models evaluating the effects of motor activity, neurogenesis, synaptogenesis, decreasing beta-amyloid, angiogenesis, and increasing brain-derived neurotropic factor (BDNF) have been observed, and these phenomena are likely to be present in the non-model, human brain.38 In addition, exercise promotes vascular health by lowering blood pressure, lipids, obesity and inflammatory markers, which are associated with risk of cognitive aggravation in AD.38 Furthermore, enlargement of the hippocampus was observed in subjects who performed systematic aerobic exercise.39 In a study with early Alzheimer's disease, Burns et al.40 found that high fitness levels were associated with less brain atrophy, resulting in preserved brain volume. In a comprehensive review, Kramer & Erickson38 reported that BDNF is an important molecule in the effects of physical exercise on the human brain and cognition. Based on experimental models, voluntary exercise increases both mRNA and protein levels of BDNF, with a favorable impact on the hippocampus, the frontal cortex, and other brain regions38–40 and increased matter integrity in select cerebral structures41, probably with a favorable impact on cognition and behavior.42 However, the neurobiological mechanisms connecting exercise and neuro-protection in patients with AD remain unclear.
In the present study, interviewers were not blinded when made data collection. The non-blinded assessment of outcome can result in an overestimation of the effects of an intervention, with the risk of apparent benefit or expectation bias. Although we recognize that blinding during data collection is a critical aspect of any controlled trial to eliminate subjective bias and improve accuracy and agree that blinding should be routinely incorporated into the design of such research, in this study, this procedure was not operationally possible. The primary reason for this was the limited availability of volunteer interviewers for the assessments. However, this problem was partially controlled for by a careful and appropriate orientation and training of the interviewers by experts on psychometric assessment and motor procedures to avoid subjective interference.
Regarding the clinical approach, physical exercise is already recommended to improve neuropsychiatric symptoms in patients with dementia and to reduce the suffering of caregivers in conducting their daily responsibilities. Furthermore, evidence supporting the favorable effects of physical exercise on neuropsychiatric symptoms has provided sufficient reason to encourage participation in motor intervention programs among patients with AD.
Our results must be interpreted with caution because there are several noteworthy methodological limitations. First, the use of non-blinded interviewers constitutes a limitation, as this methodological strategy could have weakened the reliability of the data. Furthermore, patients and caregivers were not blinded; they may have been more positive in describing their symptoms because of the effort they had put into the physical exercise during the intervention program. In addition, psychological states might have influenced the assessment of the caregivers. It is possible that their emotional and cognitive conditions have an uncontrolled impact on the interpretation of a patient's behavior. In Brazil, family members frequently provide daily care for elderly people, and the presence of professional caregivers, who would avoid psychological influences, is a not common occurrence. The absence from this study of a group of patients with severe dementia represents another limitation. Because of this deficit, it was impossible to assess the effects of physical exercise on the neuropsychiatric symptoms of severe dementia or to compare the results with mild or moderate dementia. Concerning the procedures described in the methods section, the data collection for the patient and the caregiver were completed together or separately, according to specific situations, such as emotional condition or behavior control. Although these procedures could interfere with the accuracy and quality of the measurements, the interviewers made an effort to limit the emotional influences of the patient or caregiver on the results. Although the total score on the Neuropsychiatric Inventory increased among patients in the control group, the scores in several domains on this scale slightly decreased. Whether this reduction was related to a placebo response or to natural fluctuations in the course of the disease is not clear.
Another limitation consists of the absence of psychotropic controls. The effect of these drugs was not investigated in either group in the first or second evaluation. It is possible that this could represent a bias, and our results may not be readily transferable to a clinical population. Regarding the number of patients, there was a small sample size, particularly in the control group, which substantially reduces the statistical power and capacity for the generalization of our results.
In conclusion, our results suggest that six months of aerobic exercise is associated with a reduction in the neuropsychiatric symptoms of patients with AD and contributes to the attenuation of the caregiver's burden. Irritability, anxiety, apathy, and appetite alterations were the primary improved psychopathological manifestations. The positive effects should be interpreted with caution in view of our limitations. Whether these benefits are maintained for longer periods will require further investigation.
Clinical Message- •
Aerobic exercise represents a favorable, non-pharmacological strategy for reducing behavioral disturbances of patients with AD.
- •
Caregivers whose patients participated in the exercise program presented an attenuation of burden and stress.
The authors acknowledge Etna Macário for English revision.