Aspermatogenesis is a severe impairment of spermatogenesis in which germ cells are completely lacking or present in an immature form, which results in sterility in approximately 25% of patients. Because assisted reproduction techniques require mature germ cells, biotechnology is a valuable tool for rescuing fertility while maintaining biological fatherhood. However, this process involves, for instance, the differentiation of preexisting immature germ cells or the production/derivation of sperm from somatic cells. This review critically addresses four potential techniques: sperm derivation in vitro, germ stem cell transplantation, xenologous systems, and haploidization. Sperm derivation in vitro is already feasible in fish and mammals through organ culture or 3D systems, and it is very useful in conditions of germ cell arrest or in type II Sertoli-cell-only syndrome. Patients afflicted by type I Sertoli-cell-only syndrome could also benefit from gamete derivation from induced pluripotent stem cells of somatic origin, and human haploid-like cells have already been obtained by using this novel methodology. In the absence of alternative strategies to generate sperm in vitro, in germ cells transplantation fertility is restored by placing donor cells in the recipient germ-cell-free seminiferous epithelium, which has proven effective in conditions of spermatogonial arrest. Grafting also provides an approach for ex-vivo generation of mature sperm, particularly using prepubertal testis tissue. Although less feasible, haploidization is an option for creating gametes based on biological cloning technology. In conclusion, the aforementioned promising techniques remain largely experimental and still require extensive research, which should address, among other concerns, ethical and biosafety issues, such as gamete epigenetic status, ploidy, and chromatin integrity.
Beyond the apparent anatomical simplicity of the male reproductive system, which has a basic design consisting of a pair of gonads with its corresponding excurrent ducts and associated accessory sexual glands, there lies an overwhelmingly complex system that is responsible for gamete production and transport into the female tract for ultimate sexual reproduction. Although many aspects of the endocrine regulation of testis function are well understood and therapeutic options for hypogonadal men are available, many aspects of the multiple physiological processes involved in gamete development inside the testis are often deregulated and out of homeostasis, with the subsequent outcome of absent or disturbed spermatogenesis, which leads to infertility. Human infertility is usually defined as the inability of couples to achieve pregnancy after 12 months of unprotected intercourse and is a problem that currently affects 10 to 15% of couples. An outstanding 50% of these cases are associated with male factors (1,2)
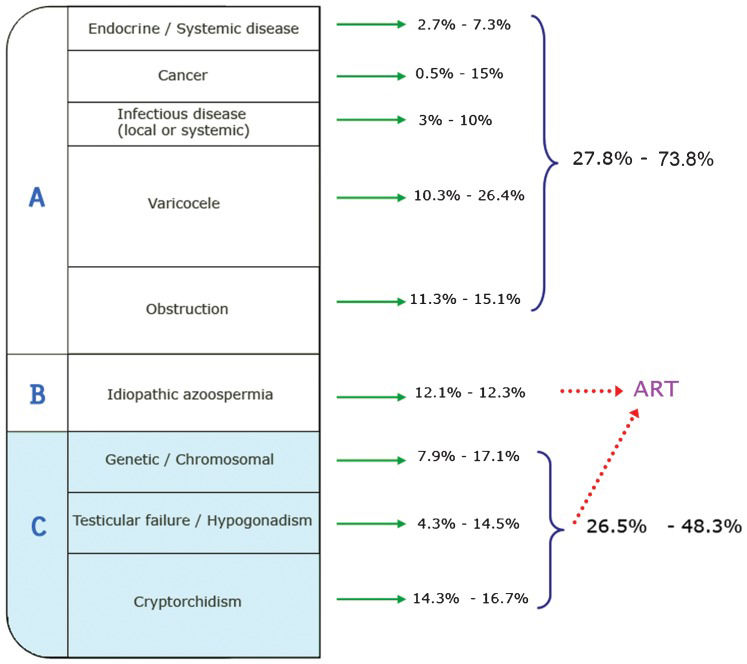
Specific etiologies of male infertility include systemic diseases (e.g., endocrine, infectious, and cancer), varicoceles, obstructive syndromes, genetic/chromosomal factors, testicular failure/hypogonadism, and cryptorchidism. Of all of the causes reported, approximately 12% of the determinants of primary dysfunction in the male reproductive organs are of unknown origin and are usually confounded by the context of multicausal origins (Figure 1).
Frequency of male infertility etiological groups determined from reproductive clinic records. Percentages for each category are presented as ranges (minimum and maximum values in the reported literature) (3,4). Patients from Group A (27.8 to 73.8% of cases) can undergo medical or surgical treatments to address their infertility problems. An unknown percentage of cases within Group B (idiopathic azoospermia) and perhaps even some from Group C can benefit from ART, provided that their sperm can be retrieved. Men suffering from aspermatogenesis, with some type of premeiotic/meiotic spermatogenic disturbance, are included in Group C.
Furthermore, it is clear that many cases of infertility are secondary to general systemic diseases or congenital defects, with reproductive consequences that may be treated when appropriate state-of-the-art procedures are implemented. Data in the literature show that 25 to 75% of cases (depending on the report/study) could theoretically be treated medically and/or surgically (Figure 1). In many cases, very few sperm are present in the testes, and they can be retrieved by biopsy and testicular sperm extraction from the tissue. Patients with access to assisted reproductive technologies (ART) can undergo ICSI (intracytoplasmatic sperm injection), which is routinely used in many fertility centers worldwide, to achieve pregnancy. However, the group of patients for whom ART procedures are not an option deserves special attention. These men present with azoospermia and varying degrees of germ cell pathology, from germinal aplasia to germ cell premeoitic/meiotic arrest. This group of testicular pathologies has a poor prognosis and holds the status of being untreatable and incurable from a medical point of view. We propose that men with incomplete spermatogenesis could be collectively classified as aspermatogenic, i.e., having a severe testicular pathology with a complete absence of spermatids. Such men comprise from 25 to 50% of andrological cases (3,4) (see Figure 1), and the only option currently available for these patients is adoption or artificial insemination using donor sperm.
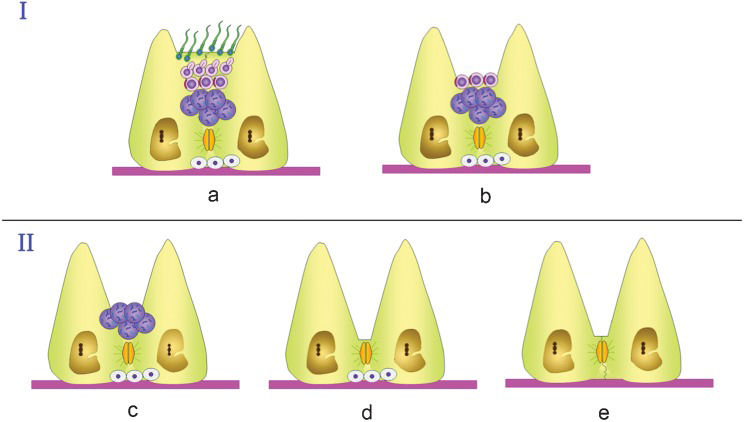
Histopathologic findings in biopsies from patients in the aspermatogenic group can be classified as: a) Sertoli cell only (SCO) syndrome, which strictly consists of testes presenting a complete absence of germ cells; b) arrest at the level of meiotic germ cells; or c) spermatogonia only (pre-meiotic arrest) (Figure 2).
Schematic summary of the types of seminiferous epithelium impairment that can lead to infertility in men. Group I summarizes pathologies in which germ cell arrest occurs at spermiogenesis. (a) Presents an apparently normal seminiferous tubule with apparently normal sperm and elongated spermatids as the most advanced cell types present. Several pathologies show a morphologically normal seminiferous epithelium, as depicted, but sperm can still present a pathological condition and therefore be unable to fertilize an egg. Many of these cases can be solved by using ART, i.e., testicular sperm extraction (TESE) combined with ICSI. (b) Arrest at the level of round spermatids, which can be used for ROSI (round spermatid injection). This type of arrest is rare. Group II represents arrest at the levels of (c) spermatocytes (12.5 % of cases) or (d) spermatogonial cells (1.7 % of cases) and (e) total absence of germ cells, as in SCO syndrome (estimated to occur in 16.5 % of cases, based on data from Tuttelmann (2010) (3), with detailed etiological entities including chromosomal aberrations, as in Klinefelter (11), XX males (12), translocations (13,14) and microdeletions at the level of the AZF regions of the Y chromosome (15,16). Prevalence data are from McLachlan et al. (2007) (5), except for the SCO data (3).
Sertoli cell only syndrome, i.e., type I SCO, refers to a situation in which testicular biopsy reveals an absence of germ cells in the seminiferous tubules (5). The presence of few seminiferous tubules showing active spermatogenesis has also been referred as to focal SCO (6) or type II SCO (MeSH database PubMed). However, in contrast to patients with complete SCO, these patients benefit from ART procedures, as their sperm can be retrieved by TESE (testicular sperm extraction). Type I SCO can have a genetically determined developmental origin that results in the disturbance of migration and/or colonization of primordial germ cells (PGCs) into the genital ridges. Because the diagnosis of type I or type II SCO depends on histopathologic analysis, which is of course prone to sampling error (because biopsies cover only a very small portion of the testis), the subtypes of SCO are often misdiagnosed. The situation would present less of a diagnostic challenge if a reliable, non-invasive method were available to distinguish cases of partial and complete absence of germ cells. The first steps in this direction involved the amplification of specific germ cells, seminal vesicles and prostate mRNA from cell-free seminal mRNA by using RT-PCR (7). Combinations of non-invasive diagnostic procedures with other procedures, such as hormonal profiles and histopathology, may unequivocally help to diagnose patients correctly. As type I SCO patients have no germ cells, the only possible way to generate offspring would be the use of their somatic cells to generate germline cells in vitro.
Arrest represents a situation in which spermatogenesis stops at a specific stage of germ cell development. McLachlan et al. proposed a nomenclature in which the term arrest exclusively refers to histological samples in which no progression occurs beyond that specific stage in any seminiferous tubule (5). Arrest can represent an intermediate step on the way to more severe germ cell losses. Arrest occurs often at the spermatocytes or spermatogonial level (12.5% and 1.7% of 534 patients, respectively) (5). Arrest during spermiogenesis is rare, and hypospermatogenesis (often misdiagnosed as arrest), in which a lower spermatid count is combined with the presence of all spermatogenic stages, which are reduced to some extent, accounts for up to 63% of cases (7). In any case, because hypospermatogenesis results in the presence of spermatids, ART procedures are applicable. Given that germ cells are present in cases of arrest, an interesting strategy would be to differentiate these cells in vitro to obtain sperm for ICSI or IVF (in vitro fertilization). Interestingly, in arrest at the level of meiotic cells, these cells can be injected into oocytes to produce viable embryos and even offspring (8-10). Nevertheless, intracytoplasmatic spermatocyte injection is only an experimental option with low efficiency. For instance, only 15% of mouse oocytes injected with secondary spermatocytes generated offspring, whereas those injected with primary spermatocytes generated none (8). Furthermore, in another set of experiments, from 0 to 9% of eggs “fertilized” by primary spermatocytes developed into adult fertile mice (9). In humans, one report showed an offspring-deriving efficiency of approximately 3% (10). For these reasons, the use of spermatocytes to compensate for a lack of more mature male germ cells will require further experimentation.
Many patients believe that biological fatherhood has a very high value. In this respect, biotechnology offers, now more than ever, a basis on which to develop clinical applications for such cases, to restore fertility and consequently to obtain offspring. In this context, advances in cell biology from several animal models are waiting to be translated into human reproductive medicine, provided that some technical issues and biosafety and ethical concerns are overcome. We propose four main groups of technologies that, alone or combined, could bring hope to men with severe cases of aspermatogenesis: i) sperm derivation in vitro; ii) germ stem cell transplantation; iii) xenologous systems; and iv) haploidization. These topics are addressed below.
SPERM DERIVATION IN VITROThe complex spatial and functional organization of the testis and its seminiferous epithelium make it difficult to achieve efficient spermatogenesis in artificial systems. The observation of at least a few aspects of spermatogenesis in culture dishes has been a scientific aim for decades (17,18). Spermatogenesis in vitro is not only a long-desired technique to gain deeper insight into the complex process of gametogenesis; it is also desired for clinical applications to broaden the therapeutic options for men with infertility problems. Given the current status of ART and the requirement of only one sperm to fertilize an oocyte through ICSI, the in vivo physiological situation, in which millions of sperm (many more than needed) are produced on a daily basis may be surpassed by in vitro strategies, which may be inefficient but which can generate a few fully mature sperm for ART procedures.
Spermatogenesis in vitro was first successfully achieved in vertebrates by using a fish model that consisted of a tissue culture system that supported full spermatogenesis in Japanese eels (19,20), which opened the possibility for similar technological advances in higher vertebrates. Systems for in vitro spermatogenesis in fish seem to be very simple from a practical point of view. For example, one such system based on a spermatogonial cell line without Sertoli cells and involving medaka fish, allowed postmeiotic advances in the presence of a culture medium containing a crude embryo extract with no specific growth factors added (21), which introduced the idea that germ cells in lower vertebrates are more intrinsically programmed and less dependent on regulation by extrinsic signals.
Currently, two main experimental approaches dominate the efforts to generate mammalian sperm in culture systems: organ culture of testes or testicular fragments that maintain the complex architecture of testicular tissue and its arrangement inside the epithelium. A recent article by Sato et al. convincingly demonstrated, in what has been described as a great scientific breakthrough, the feasibility of obtaining viable and apparently normal offspring from sperm generated through testicular tissue explants that initially contained immature germ cells (22). In this approach, the cytoarchitecture of the gonad remains widely preserved in the system, many endogenous factors are produced and released by the mostly intact seminiferous epithelium, and associated somatic cells regulate the germ cells in the system similarly to the in vivo condition. In dissected tissue, the supply of oxygen and nutrients is disturbed, and therefore the spermatogenic process is often disrupted and continues, at best, at low efficiency (22). Alternatively, enzymatically digested cell suspensions can be used for various culture systems. The supply of oxygen and nutrients to isolated cells is achieved by modern cell culture media under appropriate culture conditions. However, the seminiferous tubule microenvironment, with its different, functionally distinct compartments (e.g., interstitial, basal, intraepithelial, and adluminal), is destroyed. If these changes in the microenvironment during germ cell differentiation are significant, then spermatogenesis will be disrupted. Although many additional strategies of cell and tissue culture have been tested over the last 30 years, success has been limited (23-29). Recent studies have used monolayers other than those generated from Sertoli cells as sources of differentiating factors (30-33). Because the production of sperm in higher vertebrates involves an overwhelmingly intricate process of germ cell differentiation, further research efforts have been directed toward identifying signaling pathways associated with differentiation in the seminiferous epithelium during spermatogenesis. To incorporate this knowledge into the development of in vitro spermatogenesis systems, studies that have addressed the presence of substances such as retinol (34,35), kit ligand (stem cell factor) (36,37), insulin-like growth factor and transforming growth factor alpha (TGF-alpha) (38), as well as hormones such as testosterone (39) and FSH (40), among others, have been instrumental in recapitulating the entire differentiation process. These studies will become particularly valuable if the paradigm of spermatogenesis in vitro, as a model of the in vivo situation, is accepted. Therefore, other factors, such as scrotal temperature, the timing of cellular events and the presence of important culture media components, e.g., fetal calf serum, oxygen levels, pressure, substrates and the use of feeder layers, should be carefully evaluated and whenever possible translated from the in vivo situation to the artificial system. The use of Sertoli cells is controversial but, in principle, these cells are important for the maintenance of germ cells in culture and to provide differentiating factors (41-44). Among the advantages of cell culture systems is that such systems (cell cultures started from dissociated germ cell suspensions) are much simpler than tissue cultures, in which cell function is tightly regulated and more complex, as in the in vivo situation. Cell cultures can be started with purified, selected, isolated cells, thereby allowing uncomplicated manipulation schemes because signal redundancy, which is observed in intact biological systems, is minimized. Furthermore, as the first step of the procedure, germ cells can be stimulated to proliferate in vitro, which creates the conditions suitable to obtain large numbers of spermatogonial stem cells before inducing them to differentiate during the process of gamete production.
Recently, a novel three-dimensional testicular cell culture approach was described. The soft agar culture system (SACS), which was originally described for the culture of hematopoietic cells in vitro, has created the conditions necessary to enable the full development of murine male germ cells in vitro (45-47). This system provides options for manipulation, e.g., the testing of the effects of hormones, drugs, toxins or other compounds on germ cell development. We were able to demonstrate the progression of spermatogonia into meiosis and the cells’ further maturation into morphologically normal spermatozoa. However, the efficiency of sperm production with SACS is very low. The success of such strategies may depend on the reconstruction of small functional units of testicular cells inside the matrix, which occasionally fully resembles the microenvironment in the seminiferous tubules and thus results in small islands of complete spermatogenesis.
To recapitulate the situation in the testis, researchers must sequentially and gradually provide the right substances/conditions at the proper time, thereby emulating the in vivo processes that occur in an orderly fashion in different functional compartments (e.g., basal, meiotic, and adluminal) of the seminiferous epithelium during normal in vivo spermatogenesis. Apparently, the most challenging step of differentiation to achieve in an in vitro spermatogenic system is proceeding beyond meiosis (41). Hence, progress must be experimentally monitored by meiotic/postmeiotic-specific markers, by immunohistochemistry and/or by PCR. Some examples are summarized in Table 1. The need to distinguish somatic cells renders the use of markers for this cell group useful (Table 2).
Testicular germ cell markers.
| Cell type | Marker |
|---|---|
| Germ cell general | VASA (48) h |
| Gonocytes | ZBTB16 (49) m,h |
| Undifferentiated type A spermatogonia (single) | ID4 (50) m; CSF1R (51) m |
| Undifferentiated type A spermatogonia (single and paired) | EFR3 (52) m |
| Undifferentiated type A spermatogonia (single, paired and aligned) | THY1 (53) m; UTF1 (54) m; PLZF (55) m; LIN28 (TEX17) (56) m; NGN3 (57) m; NANOS3 (52) m; CD24 (53) m; GFRalpha1 (57) m; CDH1 (58) m; GPR125 (59) m; SOHLH2 (60) m; RET (61) m; BCL6B (62) m; SOX3 (63) m; SALL4 (64) p |
| Spermatogonia (general) | HSP60 (65) h; MAGEA-4 (66) h; UCHL1 (PGP9.5) (66) h; ITGA6 (alpha6 integrin) (66) h; ZBTB16 (66) h, (49) m,h; Kit (36) m |
| Pachytene spermatocytes | testis specific histone (TH2B)(67) r; phosphoprotein P19 (68) r; SPTRX-3 (69) h |
| Spermatocytes (general) | SCP-3 (70) b, (71) h; GRP78 (65) h; Kit (66) h; MLH1 (71) h |
| Round spermatids | Transition proteins T1, T2 (72,73) h; protamine 2 (PRM-2) (74); CREM (75) h,m; GRP78 (65) h; Kit (66) h |
| Elongated spermatids | CRES (76) h |
| Spermatids (general) | SPANX (a subset) (77) h; KLF4 (78) h; SPTRX-3 (69) h; SP-10 (79) h; TGFbeta3 (80) h |
| Sperm | outer dense fibers (ODF-2) (70); acrosin (81) b; SPANX (77); HSP60 (65) h; GRP78 (65) h; CRES (76) h; DAZ2 (82) h |
m, mouse; r, rat; d, dog; b, bovine; p, non-human primate; h, human.
Testicular somatic cell markers.
| Cell type | Marker |
|---|---|
| Sertoli immature | AMH (anti-mullerian hormone) (83) p; WT1 (Wilms’ tumor gene)- transcription factor (83) r; aromatase (P450 enzymes) (83) r; NCAM (neural cell adhesion molecule) (83) r; cytokeratin 18 (83,84) h; M2A (83) h |
| Sertoli mature | occludin (85) m,r,d; vimentin (86) r, (43,87) b; P27Kip 1 (cyclin-dependent kinase inhibitor) (83) m,r,h; WT1 (Wilms' tumor gene)- transcription factor (83) r; Dmrt1 (88) m; Gata 4 (88) m; Gata 1(83) m; AR (androgen receptor) (83) r,p,h; transferrin (88) r; ITGA6 (alpha6 integrin) (66) h |
| Leydig cells | ITGalpha6 (alpha6 integrin) (66) h; RLF (89) m; 3 beta-HSD (90) g, (91) r; TGF alpha (92) r |
| Peritubular myoid cells | alpha-smooth muscle actin (93) r, (43,87) b |
m, mouse; r, rat; g, guinea pig; d, dog; b, bovine; p, non-human primate; h, human.
These findings have greatly inspired further research involving humans in the hope of reversing cases of incomplete spermatogenesis. This type of approach would require biopsy procedures, which usually yield small testicular samples with limited numbers of germ cells. If the availability of testicular tissue is not an obstacle, then fertility patients with immature germ cell arrest could expect to overcome infertility by using this procedure. Moreover, cell culture systems for in vitro spermatogenesis would provide advantages not only to germ cell arrest patients but also to type II SCO syndrome cases or cases with other reproductive pathologies involving faulty spermatogonial stem cell niches.
Current technology allows, at least on the theoretical level, SCO syndrome patients, who have no germ cells, to achieve the goal of producing offspring that retain their own genetic material. To this end, biotechnology must first provide gametes to help these men overcome their fertility problems. Pluripotent stem cells could be a reliable and plentiful source of immature germ cells to provide a baseline for a differentiation process that eventually will yield spermatozoa in vitro. Pluripotency is the property of a cell to differentiate into somatic cells of ectodermal, endodermal and mesodermal lineages, which constitute the vast majority of the cells in the body (94). It has been demonstrated that some pluripotent stem cells can also generate germ cells. Pluripotent stem cells can be basically obtained from two sources: a) the epiblast region in blastodermic embryos, and b) adult somatic cells. The latter source provides an opportunity for success in the great challenge of obtaining pluripotent stem cells from adult patients, despite the conventional wisdom in cell biology that cells in tissues tend to progress into lineage restrictions during development, gradually reaching terminal differentiation. The idea of somatic pluripotentiation, or reprogramming, has been discussed for quite some time and found original success in mouse models (95). Efforts continued shortly thereafter using human cells (96,97). Only recently, independent research groups (98,99) went a step further to achieve the major breakthrough of defining four basic pluripotency-inducing factors, i.e., Oct4 (pouf1), Sox2, c-Myc, and Klf4, which are required to switch somatic cells into a pluripotent state, thus opening a window for deeper understanding of pluripotency networks, as well as the possibility of generating germ cells, among other cell types, from somatic cells induced to be pluripotent (iPS cells). The availability of such personalized germ cells of somatic origin would be a prerequisite for an in vitro sperm-generating system for SCO patients.
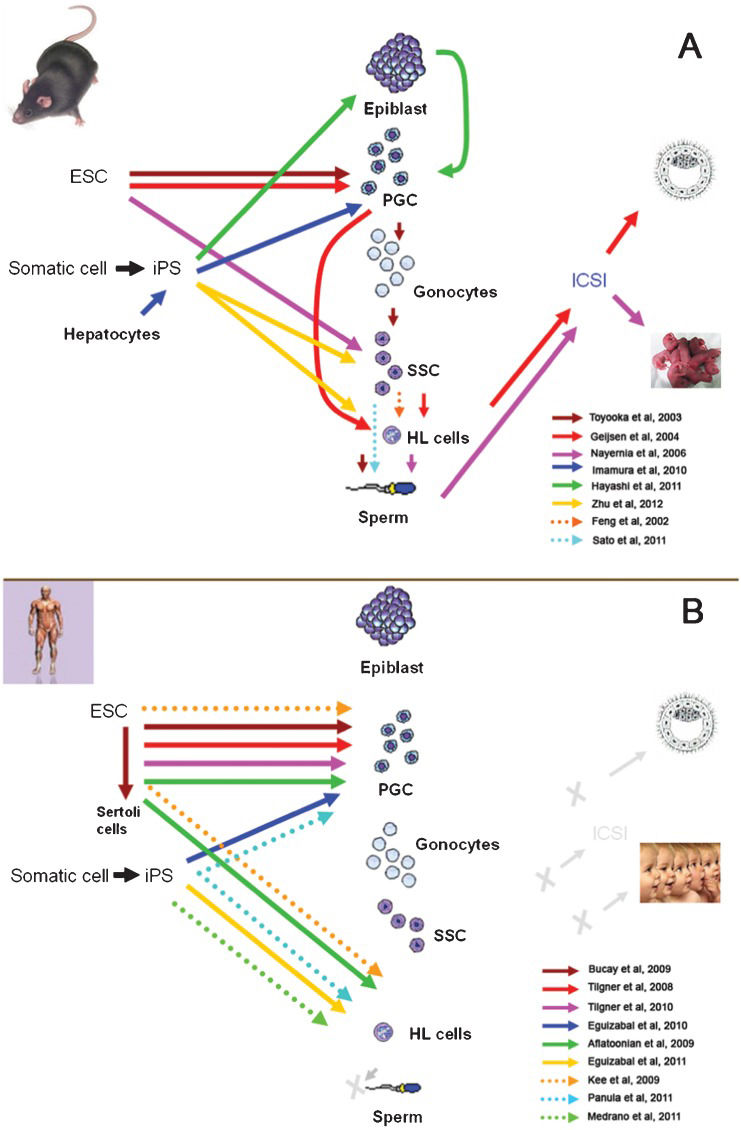
The development of in vitro spermatogenesis has benefited from various animal models, including models that use farm animals. Some culture systems were developed in domestic animal models in which germ cells progressed to advanced steps of spermatogenesis. For instance, Izadyar et al. observed elongated spermatids in a long-term culture of bovine germ cells (70), whereas Dong et al. obtained elongated spermatids from fetal calf gonocyte cultures (100). In these species, the available procedures proved to be rather inefficient because not many terminally differentiated gametes could be derived. In parallel, murine models involving ES (embryonic stem) cells resulted in advances in the field. In principle, germ cell derivation from ES cells does not seem to be practical for infertility patients, as almost no man has access to his own embryonic cells. However, because iPS cells appear to be similar to ES cells (101), and in nature, technically derivable from somatic cells, the resulting scenario becomes very promising to drive them into the production of gametes in vitro. Efforts to generate germ cells and gametes from ES cells and iPS cells in murine models and humans are summarized on Figure 3.
Recent advances in the derivation of germ cells / gametes from ES cells and iPS cells in mouse models (A) and in humans (B). ESC, embryonic stem cells; iPS, induced pluripotent stem cells; PGCs, primordial germ cells; SSC, spermatogonial stem cells; HL, haploid-like cells; ICSI, intracytoplasmatic sperm injection. In (A) arrows point into the cell types generated by each specific research group. Grey letter X and arrow in (B) indicate advances not yet accomplished.
Briefly, three research groups have made advances in the derivation of germ cells from mouse ES cells. One of these groups produced gamete-like cells, and using ICSI as a functional assay, they managed to obtain embryos after fertilization had reached the blastocyst stage (102). Two laboratories were able to generate sperm in vitro (31,103), but only one of them obtained offspring by injecting the derived sperm into donor oocytes using ICSI (31). However, in this case, the offspring production had low efficiency (7 out of 65 embryos), and the mice that were born died prematurely (five months after birth), which suggests developmental aberrations. Progress in germ cell derivation from mouse iPS cells was initially more limited because none of the groups involved could obtain offspring, although PGCs were obtained in one case (104) and gamete-like cells were also recently obtained (105). Recent advances have also been made in humans. PGCs were derived from human ES cells by several groups (30,106,107). Recently, Aflatoonian et al. not only generated PGCs but also derived human sperm-like cells (haploid-like cells) for the first time (108). In another recent study, haploid-like cells were produced from human iPS cells (33). Nevertheless, up to now, no human embryos have been generated using these techniques, and the scientific community continues to work together with clinicians to develop rigorous, ethical and secure tests and protocols for these innovative techniques.
The principles underlying these methods are simple enough to allow further progress from experimental to applied status. In summary, pluripotent ES cells are first given a differentiation environment in vitro to produce multiple cell lineages, and then, in a second phase, cells are selected for specific antigen expression or reporter germ cell genes. Also important is the addition of differentiating factors. The current stage of methods for the derivation of human haploid-like cells in vitro allows the generation of gametes with the correct epigenetic status (32,109,110). Taken together, all of the findings presented are genuine biotechnological advances with potential applications in cases of men who present with aspermatogenesis, but regardless of the tremendous potential of these techniques for the derivation of germ cells to produce gametes in vitro using culture techniques, much research remains to be conducted in this area.
GERM STEM CELL TRANSPLANTATION AND XENOLOGOUS SYSTEMSIn cases in which the testes of infertile men contain spermatogonia, the stem cell fraction among these premeiotic germ cells could be targeted for fertility preservation. The presence of male germline stem cells indicates high regenerative potential, as these cells can efficiently recolonize the seminiferous tubules, even when the starting population of spermatogonial stem cells is small. When the technique was first applied by Brinster and Zimmermann in 1994 (111), a crude, single-cell suspension containing spermatogonia was infused into the testis. The stem cells migrated into testicular stem cell niches spontaneously, whereas somatic and more differentiated germ cells were flushed out. Recolonization was initiated from individual spermatogonia, which slowly but steadily divided and migrated to repopulate all of the seminiferous tubules (111,112).
The technique of germ cell transplantation could, in principle, be applied across species. Microinjection of germ cell suspensions from rats into the seminiferous tubules of mice led to the initiation of spermatogenesis from donor spermatogonial stem cells. Spermatogonia from less closely related species have the ability to repopulate the testis but will not differentiate, which currently renders the production of primate sperm in mouse testes impossible. Subsequent studies in rodents showed that spermatogonia can be cryopreserved and expanded by in vitro culture prior to germ cell transfer. The clinical potential of this technique was shown in non-human primates and in experimental studies using human testes. Ultrasound-guided infusion of germ cell suspensions via the intratesticular rete testis appears to offer a simple and relatively non-invasive procedure for the autologous infusion of germ cells back into the patient (113). Our preclinical studies have demonstrated the feasibility of transplanting germ cell suspensions into the testes of non-human primates and men (113,114). A first clinical trial was initiated at the Christie Hospital in Manchester, UK, in 1999. The fertility outcomes of the patients in this study have not been yet published (115,116). In conclusion, important issues in achieving re-fertilization through germ cell transplantation include the retrieval of sufficient testicular tissue or in vitro expansion strategies for spermatogonia, prevention of ischemia in the dissected tissues and cells, cryopreservation and thawing of cell suspensions and sorting of tumor cells or enrichment of spermatogonial stem cells. The only issue solved is the ultrasound-guided non-invasive transfer of germ cell suspensions into the rete testis. These critical steps must be optimized by future research before this technique can offer a promising and safe option for the protection of male fertility in men with spermatogonial arrest.
Testicular grafting is an extreme form of organ culture in which the tissue is transplanted into a host. The environment is well controlled, and also the blood supply to the grafted tissue is restored. Immature testicular tissue has a high regenerative capacity and can survive and develop as an auto- or xenograft. In the absence of alternative strategies to generate sperm in vitro, grafting provides an approach to ex-vivo generation of mature sperm. In mice, sperm generated in grafts were capable of generating healthy offspring. We and others have recently shown that xenografting of neonatal and prepubertal testicular tissue from a variety of species permitted the generation of sperm, whereas xenografting of more developed tissue resulted in the degeneration of testicular tissue. We have also shown that immature primate testes can be maintained on ice for 24 hours prior to xenografting and can also be cryopreserved. Grafting will create exciting, clinically applicable strategies for fertility preservation in immature patients. However, fertility problems are usually diagnosed in adulthood, when grafting appears to be a less useful tool for generating sperm. We therefore consider the application of grafting techniques to be more valuable for fertility preservation in children undergoing oncological treatment.
An alternative to achieve germ cell development is the use of xenologous systems, which recapitulate the organogenesis of a functional testis [for review: Gassei and Schlatt, 2007, (117)]. The male pathway involves regulated cell differentiation of somatic cells within the gonadal primordium, including the migration of mesonephric cells and primordial germ cells. Finally, testis cords form and begin to elongate. Tissue engineering approaches that mimic male embryonic gonadogenesis could offer novel ways to study early testicular differentiation (29) and may, when eventually combined with xenografting approaches, also provide scenarios for the differentiation of male germ cells.
HAPLOIDIZATIONIn previous sections, several prospective technologies were proposed to overcome the lack of gametes in men with aspermatogenesis. These technologies are based on differentiating preexisting or pluripotential stem cell-derived germ cells in vitro (spermatogenesis in vitro) or on in vivo systems (transplantation and xenografting) to produce sperm.
Haploidization is an alternative approach to manufacture gametes based on biological cloning technology. In cloning, an entire genome is obtained from a single individual when he donates a somatic cell nucleus, which is transferred into an enucleated, developmentally young cell (MII oocyte, zygote, embryo) (118). In contrast, haploidization is a modified cloning technique in which there is a genetic haploid contribution from both parents, which is one reason why this technique has also been called semi-cloning (119,120).
Although promising, this technology has not been successful for generating live normal offspring in murine experimental models, and therefore it remains largely experimental, with no immediate prospects for clinical application. For instance, in preliminary experiments, only 17 to 22% of mouse oocytes “fertilized” with somatic cells formed blastocysts, and no offspring were derived from them (121).
EXPERT COMMENTSThis review article provides an overview of several biotechnological approaches that have the potential to offer solutions for a range of reproductive pathologies that cause sterility in a significant number of azoospermic men, while at the same time providing new tools to contribute to our current knowledge of male reproductive physiology. Although they are thus far experimental, the proposed emerging technologies could work in combination with other already well-established reproductive technologies and take advantage of the requirement for just one healthy spermatozoon to ensure successful fertilization and, hopefully, normal offspring from these individuals.
Thus far, only haploid-like cells, with an apparently correct epigenetic status, have been generated by several research groups. In the near future, we can expect the generation of sperm as the most natural, terminally differentiated cells to be used in assisted reproduction for men afflicted with aspermatogenesis.
Regardless of the pervading optimism, remaining challenges include the verification of aspects such as normal cytogenetics, epigenetic stability, the tumorogenic potential of derived germ cells and even ethical aspects, which should guarantee the correct implementation of these new reproductive technologies. Additionally, the genetic basis for many conditions of aspermatogenesis will necessitate caution, as well as a consideration of the need to develop gene therapies that are specific for these reproductive problems to prevent their transmission to future generations.
KEY ISSUES•At least 25% of azoospermic men are sterile because their testes either hold only immature or no germ cells at all, a condition that can be collectively termed as aspermatogenesis.
•Possible solutions to aspermatogenesis require biotechnological approaches to retain biological fatherhood.
•Azoospermic men with germ cell arrests at early stages of spermatogenesis would benefit from sperm derivation in vitro or ex vivo.
•Azoospermic men with type II SCO would have the alternatives of germ cell derivation from a) somatic cells via induced pluripotency or b) haploidization.
•The most challenging differentiation step in an in vitro spermatogenic system is completion of meiosis which has been achieved in several animal models by ex vivo approaches or 3D culture systems.
•In the absence of alternative strategies, testicular grafting (auto- or xenograft) should be considered to ex-vivo generate mature sperm and would be more valuable for fertility preservation in children undergoing oncological treatment.
•Haploidization is a modified cloning technique, in which each parent genetically contributes with a haploid complement, but unfortunately this technique offers no immediate perspective for clinical application.
•Although extensive research has been and continues to be performed, biotechnologies described on this review article still remain largely experimental.
This study was supported by CNPq and FAPEMIG.
No potential conflict of interest was reported.
All of the authors who are listed participated sufficiently in the present review article and therefore take public responsibility for its content.