BOLD MRI contrast results from changes in the microvascular ratio of oxyhemoglobin (oxyHb) to deoxyhemoglobin (deoxyHb) (1). DeoxyHb is paramagnetic, which results in a local bulk magnetic susceptibility effect and subsequent MRI signal change in T(2)∗-weighted functional MRI scans (1). Although BOLD contrast has been studied extensively in functional MRI studies of the brain, limited investigation has been performed in other tissues.
Juvenile idiopathic arthritis (JIA) is the most common rheumatic disorder of childhood (2), with the knee being the most frequently affected joint (3). With the increasing availability and use of disease-modifying and antirheumatic biologic drugs for the treatment of JIA (4), the early determination of joint abnormality has become extremely important. Given the lack of clinical and laboratory tests that enable the functional evaluation of perisynovial oxygenation in JIA, the use of BOLD MRI to measure early soft tissue physiological imbalances due to hypoxia is appealing (5).
In inflammatory arthritis, the increased metabolic demand of the inflamed synovium and the inadequate delivery of oxygen caused by poor perfusion through the inflamed synovium (5) result in hypoxia within the inflamed joint in the acute stage of arthritis. Consequently, the synovial blood flow increases to compensate for the hypoxic status of the joint. In JIA, despite the local hyperemia, the synovial capillaries develop an abnormal oxygen transportation capability (5,6), which may result in abnormal concentrations of oxyHb and deoxyHb at the capillary level. To date, no single imaging assay or surrogate marker has been demonstrated to adequately reflect the spectrum of metabolic events involved in inflammatory arthritis. We hypothesized that BOLD MRI would be able to reflect the state of oxygenation in the microcirculation of periarticular tissues in JIA children at 1.5 Tesla as previously shown for other pathological states (7).
Previous studies of our group in rabbit models of inflammatory arthritis demonstrated the feasibility (8), criteria validity (9), interframework reliability for data acquisition (10), combination of region-of-interest (ROI)-related reading parameters that provide the highest accuracy for discrimination of the presence or absence of arthritis in acute and subacute stages of the disease (11), and responsiveness of the technique to short-term joint temperature changes (12). However, no previous study has demonstrated whether the BOLD MRI technique is able to differentiate inflammatory from healthy perisynovial tissue at 1.5 Tesla and determine interval soft tissue changes as a result of intraarticular corticosteroid injections in the knees of JIA patients. In the present paper, we describe the BOLD MRI methodology and preliminary results on the feasibility of using BOLD MRI at 1.5 Tesla to measure the responsiveness (sensitivity to change) of the interval of soft-tissue changes in JIA patients with unilateral knee arthritis following intraarticular injection of corticosteroids as a proof of concept. We knew in advance the expected effectiveness of the injection procedure, which reduces synovial inflammation in JIA in approximately 76% of cases at 6 weeks after the injection (13).
MATERIALS AND METHODSThe study protocol was approved by the Research Ethics Committee of our institution. All patients' parents or the patients themselves provided informed consent for participation of their children or themselves in the study. Seven (males, 2; females, 5) JIA (subtypes: systemic, 1; polyarticular, 2; oligoarticular, 4) patients with ages ranging between 6 and 16 (median, 9) years at the time of the MRI examination were recruited from the Department of Rheumatology of our institution for the study based on acceptance for participation within the pool of patients who fulfilled the study inclusion criteria. Four (57%) patients had activity in the left knee, and 3 (43%) had activity in the right knee. The median (range) duration of disease prior to the MRI scan in the evaluated patients was 36 (1–60) months. All patients had clinical signs of unilateral knee arthritis and a clinical indication for intraarticular corticosteroid injection. The involvement of only one of the knees in each study patient was confirmed by performing MRI on both knees because MRI would be able to demonstrate asymptomatic involvement of the contralateral knee. The patients were imaged immediately prior to and 45 days after the procedure. Patients with any previous history of arthritis in the unaffected knee, with chronic pulmonary disease, or who required sedation for performance of the MRI examination were ineligible for the study.
Ten (males, 7; females, 3) healthy control children (median age, 14 years; range, 10–18 years) were imaged at a single time point using the same MRI protocol used for the JIA patients.
Imaging AcquisitionThe imaging was performed on a GE LX 1.5T MR magnet (General Electrics Healthcare, Milwaukee, WI, USA) with a maximum gradient strength of 2.2 Gauss/cm using an extremity coil. Both knees of the JIA patients and control subjects were imaged. Each knee was scanned separately. A sagittal gradient-echo localizer was used to localize the suprapatellar recess of the knee. A multiecho axial T2 susceptibility-weighted sequence (slice thickness = 5 mm, TE = 40 msec, TR = 2000 msec, bandwidth = 62.5 KHz, NEX = 1.0, and FOV = 17 cm) was applied, with 30-second periods of normoxia and 30-second periods of hyperoxia (100% O2 through a face mask at the rate of 15 L/min using a Laerdal bag with an oxygen reservoir and a one-way valve). The scan time for this sequence for each knee joint included 2 min of scan time under normoxia and 2 min under hyperoxia. A T1-weighted two-dimensional spin-echo sequence was used for anatomical localization of the BOLD data (8 contiguous axial slices, 17-cm FOV, 4-mm thickness, TE = 10 ms, TR = 500 ms, 2 NEX, 160 phase-encoding steps, and a 256x192 matrix).
Imaging AnalysisAnatomic images were aligned with corresponding functional images to enable the delineation of ROIs involving the synovium of the knee. Statistical reactivity maps were then constructed with Stimulate software (University of Minnesota Medical School, Minneapolis, MN). The maps interpolated the 64x64 functional images to overlay them onto the anatomic images. The maps provided information about the number of voxels with r-values above a pre-established threshold [expressed as a percentage of the suprathreshold voxels in the ROI (% of activated voxels)] and about the t score, indicating the difference between the on and off signal intensities that represent the activated and baseline states (‡R2∗) divided by the error of signal difference (on-off difference) for the knees at each time point. Graphically, the hyperoxic stimulus and normoxia were represented by squared peaks (on-signal intensity) and baseline squared curves (off-signal intensity), respectively. We used the 0.01 threshold with a 100% ceiling scale of 1, a positive activation algorithm, and a small ROI (11) for this study. A single operator (N.C.) traced freehand-drawn ROIs involving the immediate 0.5 cm from the bone contour of the proximal tibia and distal femur, which involved the perisynovial region.
Statistical AnalysisWe used a two-sample Student t-test to compare the baseline (pre-injection) % of activated voxels and on-off differences of BOLD MRI measurements between arthritic and contralateral non-affected knees in JIA patients and between the contralateral knees of JIA patients and control knees of healthy subjects.
BOLD MRI results were reported as the mean±standard error (SE).
The baseline and 6-week post-injection BOLD MRI measurements in the arthritic and contralateral non-affected JIA knees were compared with paired Student t-tests.
Differences with a two-tailed p-value of less than 0.05 were considered to be significant.
RESULTSAll subjects in this study tolerated the BOLD MRI procedure well. One of the JIA patients underwent the baseline procedure but, for reasons unrelated to the study, could not participate in the 6-week imaging assessment. We report the baseline results for 7 JIA patients and 10 control subjects and the 6-week follow-up results for 6 JIA patients.
The numerical values of the BOLD MRI measurements obtained in arthritic knees at baseline (prior to corticosteroid injection) were greater than the numerical values obtained in the contralateral non-affected knees of the JIA patients (Figure 1). This result was noted either using the % of activated voxels [mean value for arthritic and contralateral knees, 12.14 (SE, 2.11) and 9.61 (SE, 0.88), respectively] or on-off differences [mean value for arthritic and contralateral knees, 2.71 (SE, 0.47) and 2.28 (SE, 0.11), respectively]. Nevertheless, there were no significant differences between the measurements of the arthritic and contralateral non-affected knees in terms of the % of activated voxels (p = 0.20) or on-off differences (p = 0.21).
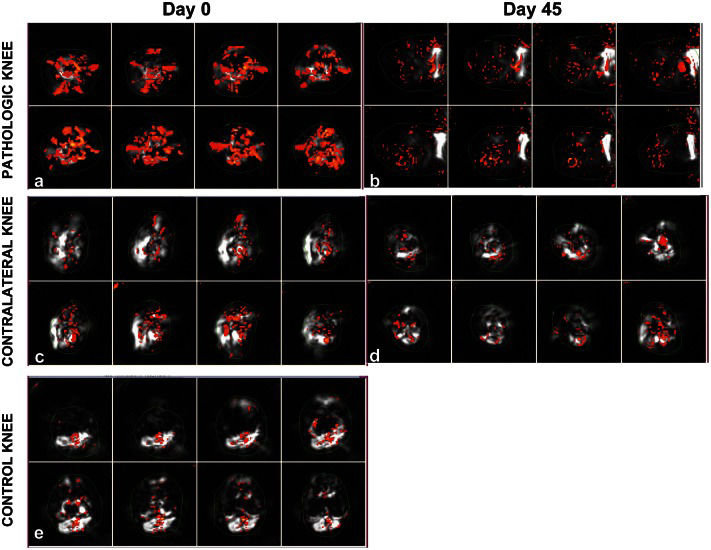
Axial BOLD MR images of arthritic (a, b) and contralateral (c, d) knees of a 7-year-old female JIA patient obtained prior to (a) and 6 weeks after (b) intraarticular corticosteroid injection. The thin green lines delineate the ROIs used to derive reactivity maps for the perisynovial regions. Note the increased percentage of activated voxels representing increased numerical values for BOLD MRI measurements observed in the arthritic knee prior to local treatment (a) and its interval decrease (b) following treatment. Axial BOLD MR images of one knee of a healthy 14-year-old male volunteer (e) show synovial reactivity represented by activated voxels similar to those of the contralateral knee of the JIA patient.
Conversely, the numerical BOLD MRI values measured in the contralateral non-affected knees of the JIA patients matched those obtained in the control subjects. The mean % of activated voxels in the contralateral non-affected knees of JIA children at baseline was 9.61 (SE, 0.88), and the value was 9.54 (SE, 1.97) in the control knees. With regard to on-off differences, the mean value measured in the contralateral non-affected knees of JIA patients at baseline was 2.28 (SE, 0.11), and the value was 2.43 (SE, 0.36) in the control knees. No significant differences were noted between the contralateral non-affected knees and control knees using either the % of activated voxels (p = 0.97) or on-off differences (p = 0.61).
Regarding the relation to the sensitivity to change of the BOLD MRI between pre-injection and 6-week-post-injection measurements in the JIA arthritic knees, the values decreased over time, but the differences were not significant at the pre-established alpha level [% of activated voxels: pre-injection, mean, 13.05 (SE, 2.95); 6-week-post-injection, 8.02 (SE, 1.08), p = 0.16; on-off differences: pre-injection, mean, 2.79 (SE, 0.75); 6-week-post-injection, 2.25 (SE, 0.33), p = 0.53].
The BOLD MRI measurements over time in the contralateral non-affected knees of JIA patients presented smaller numerical interval differences than those presented by the arthritic knees, but the differences were not significant. The mean % of activated voxels measured in the contralateral non-affected knees of the JIA patients at baseline was 11.57 (SE, 2.30), and at 6 weeks, the value was 9.43 (SE, 1.74, p = 0.08). The mean on-off difference value measured in the contralateral knees at baseline was 2.66 (SE, 0.50), and at 6 weeks, the value measured was 2.53 (0.28, p = 0.76).
DISCUSSIONThe results of this study indicate that the BOLD MRI values were higher in the knees with active inflammation than in contralateral unaffected knees, and the interval pre- and post-injection changes were greater in the arthritic group than in the contralateral joint group (Figure 1). As this was a pilot study in humans with a small sample size, no significant results were obtained. Therefore, we were unable to demonstrate the feasibility of the technique at 1.5 Tesla and suggest further investigation of BOLD MRI for arthritis with higher field strength scanners.
Compared with conventional 1.5 Tesla MRI units, high-field-strength MRI scanners operating at 3 Tesla offer the advantage of a 3- to 4-fold higher signal-to-noise ratio (SNR) (14), which is essential for imaging techniques, such as BOLD MRI, that yield minimal changes (15) when conducted using conventional 1.5-Tesla MRI scanners. At 1.5 Tesla, BOLD imaging results in signal changes on the order of 1–2%, whereas at 3 T, the signal change increases to 3–5% (14). Given the large variability of BOLD MRI measurements, as shown in this pilot study, this technique is less suitable for clinical use at 1.5 Tesla. Considering that both the SNR and the magnitude of changes in the BOLD signal can be increased with the use of MR magnets with increased field strength (3 Tesla and higher), this technique merits further investigation at higher MRI field strengths.
The chief limitation of this study is its small sample size, which is related to its status as a pilot study. The sample size was opportunistic. Furthermore, no age- or gender-matching was possible within the limitations of the small sample size of the study. Nevertheless, documentation of the preliminary results of this study enables other groups to carefully consider how to conduct similar clinical arthritis experiments in the future. In addition, our study encourages the pursuit of further investigation of hypoxia-related changes in arthritis using BOLD MRI at 3 Tesla or higher field-strength magnets. In such future studies, the power/sample-size estimates can be calculated based on the preliminary data as well as SNR calculations of BOLD MRI data.
Previous studies have demonstrated that the BOLD MRI signal represents oxygen extraction, which relates to local synovial hyperemia as a compensatory mechanism for hypoxia (9). This technique can thus be complementary to dynamic contrast-enhanced MRI, which can also provide information on synovial vascular permeability and tissue perfusion (16). However, BOLD MRI has the advantage of not requiring intravenous contrast injection.
If future clinical trials in larger samples of JIA patients at 3 Tesla MRI demonstrate the value of BOLD MRI for assessing hypoxic conditions in JIA joints, this technique may be a valuable tool to provide guidance and follow-up for systemic therapies using biological and disease-modifying agents.
AUTHOR CONTRIBUTIONSDoria AS contributed to the intellectual design, data acquisition, interpretation of results, statistical analysis, manuscript draft writing, draft review, and project overview. Crawley A and Babyn P contributed to the intellectual design, interpretation of results, and draft review. Rayner T contributed to the data acquisition and draft review. McLimont M and Laxer R contributed to the patient recruitment and draft review. Moineddin R contributed to the statistical analysis and draft review. Feldman B contributed to the intellectual design, patient recruitment, and draft review.
We would like to thank Mr. Niels Celeghin for performing the BOLD MRI data analysis and Dr. Edson Amaro Jr. (InRad, USP) and Dr. Stephen C. R. Williams (King's College London) for providing the idea for this project. We would also like to thank Dr. Claudio Campi de Castro (InCor, USP) for his support for this study at its early stage at InCor, USP.
No potential conflict of interest was reported.