The purpose of this study was to evaluate the effectiveness of contrast-enhanced ultrasound with a second-generation contrast agent in distinguishing between occlusion and pseudo-occlusion of the cervical internal carotid artery, comparing it with that of conventional Doppler ultrasound and the gold standard, computed tomography angiography.
METHOD:Between June 2006 and June 2012, we screened 72 symptomatic vascular surgery outpatients at a public hospital. Among those patients, 78 cervical internal carotid arteries were previously classified as occluded by Doppler ultrasound (without contrast). The patients were examined again with Doppler ultrasound, as well as with contrast-enhanced ultrasound and computed tomography angiography. The diagnosis was based on the presence or absence of flow.
RESULTS:Among the 78 cervical internal carotid arteries identified as occluded by Doppler ultrasound, occlusion was confirmed by computed tomography angiography in only 57 (73.1%), compared with 59 (77.5%) for which occlusion was confirmed by contrast-enhanced ultrasound (p>0.5 vs. computed tomography angiography). Comparing contrast-enhanced ultrasound with Doppler ultrasound, we found that the proportion of cervical internal carotid arteries classified as occluded was 24.4% higher when the latter was used (p<0.001).
CONCLUSIONS:We conclude that, in making the differential diagnosis between occlusion and pseudo-occlusion of the cervical internal carotid artery, contrast-enhanced ultrasound with a second-generation contrast agent is significantly more effective than conventional Doppler ultrasound and is equally as effective as the gold standard (computed tomography angiography). Our findings suggest that contrast-enhanced ultrasound could replace computed tomography angiography in this regard.
Extracranial carotid artery stenosis can cause neurological symptoms such as stroke (brain attack), which is one of the leading causes of morbidity and mortality worldwide 1. Carotid stenosis causes cerebral microembolization to the brain and occlusion is usually interpreted as the cessation of the risk of embolic events; thus, no surgical treatment is required. However, in rare cases (2-8% of cases annually), carotid occlusion or pseudo-occlusion continues to cause neurological symptoms, despite the appropriate medical treatment 2.
Although digital subtraction angiography (DSA) is the gold standard method for evaluating stenosis of the internal carotid artery (ICA), the procedure is invasive and is associated with a considerable risk of post-procedure neurological complications 3. Previous reports have established that computed tomography angiography (CTA) is nearly as accurate as DSA and can also be considered as the gold standard for evaluating such cases 4-6. However, CTA is not without adverse affects, given that it involves the use of ionizing radiation and iodinated contrast media 3.
Conventional ultrasound (Doppler US) is 95-99% accurate for detecting ICA stenosis, even when there is a high degree of stenosis 7,8. However, when evaluated by Doppler US, some symptomatic patients with near-occlusion stenosis, usually associated with very low flow, are wrongly classified as having occlusion 9,10. The establishment of a safer, simpler, cost-effective and readily available imaging methods capable of more accurately confirming or excluding a diagnosis of occlusion would facilitate decisions regarding the ideal treatment 2,7. Contrast-enhanced ultrasound (CEUS) has been shown to be effective in distinguishing between occlusion and pseudo-occlusion of the ICA 6,9,. Microbubble contrast agents substantially increase the ultrasound reflectivity in the bloodstream, thus increasing the diagnostic accuracy 15. The first generation of such contrast agents comprised microbubbles that were filled with air and were consequently very short-lived, which limited their clinical utility 16,17. The second generation of microbubble contrast agents used inert gases such as fluorocarbons, which are more stable, thereby prolonging the lifespan of the microbubbles in the bloodstream (≤10 min) and improving the sensitivity of the ultrasound 16,17. Microbubbles filled with such gases have diameters ranging from 1 to 10 µm 16,17, approximating the diameter of a red blood cell (7 µm).
The main objective of this study was to evaluate the effectiveness of CEUS with a second-generation contrast agent in distinguishing between occlusion and pseudo-occlusion of the cervical ICA, comparing it with that of Doppler US. To test the diagnostic power of the CEUS approach, we also compared it with CTA.
MATERIALS AND METHODSThis was a prospective study conducted between June 2006 and June 2012 at the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HCFMUSP, University of São Paulo School of Medicine Hospital das Clínicas) in the city of São Paulo, Brazil. The study was approved by the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo Research Ethics Committee.
The study sample comprised consecutive patients with suspected ICA occlusion followed at the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo Vascular Surgery Clinic and referred for confirmatory diagnosis between June 2006 and June 2012. We screened 72 symptomatic vascular surgery outpatients treated at HC-FMUSP who presented with 78 ICAs that were previously classified as occluded by Doppler US (without contrast) at another facility.
Those patients were again examined by Doppler US, as well as by CEUS and CTA. Doppler US was performed by a radiologist with extensive training in the use of this imaging method. CEUS, aimed at identifying pseudo-occlusion by studying the residual lumen, was performed by that same radiologist. CTA was used as the reference standard and the results were evaluated by two other radiologists, each with ten years of experience, both of whom were blinded to the evaluation made by the other, as well as to the CEUS results. When there was disagreement between the two CTA evaluators, the diagnosis was made by consensus.
UltrasoundFor Doppler US and CEUS, we used high-resolution ultrasound instruments (HDI 5000 and IU 22; ATL-Philips Medical Systems, Bothell, WA, USA). Depending upon the biotype and the anatomy of the cervical carotid arteries of the patient, we used linear array transducers (7.0-12.0 MHz) or convex array transducers (3.5-7.0 MHz). The processing parameters of the ultrasound device were exclusively adjusted to evaluate the specific peripheral vessels of the cervical carotid arteries in the Doppler US and CEUS examinations. To optimize the acoustic properties of the contrast agent in CEUS, we used tissue harmonic imaging and pulse inversion harmonic imaging. Patients were evaluated in a supine position with extension and contralateral rotation of the head toward the carotid segment being studied. We thoroughly evaluated the ICA from its emergence and bifurcation to the most distal ends, using linear or convex transducers as required.
In the initial evaluation of the ICA, as well as in the investigation of the presence of material occupying the vessel lumen, we used Doppler US in B mode. To quantify the blood flow within the ICA, we used conventional color and power color Doppler US, as well as pulsed Doppler US (Figure1).
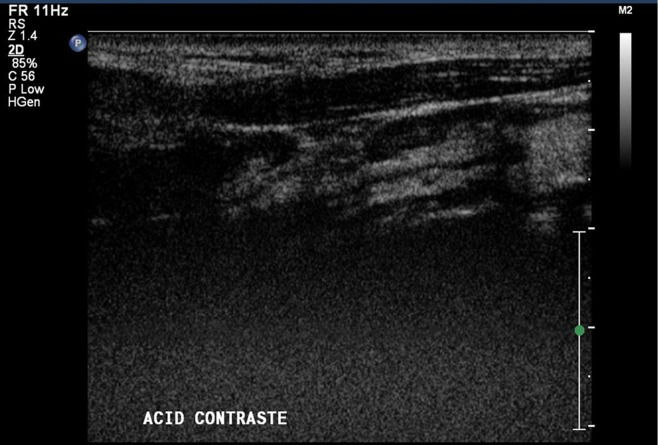
In the CEUS, we employed the second-generation microbubble contrast agent perfluorocarbon-exposed sonicated dextrose albumin (PESDA), manufactured in the Radiology Laboratory of the FMUSP. We chose to use our own PESDA rather than a commercial contrast agent to minimize costs. We produced PESDA using the technique described by Porter et al. 18. In brief, a solution of 5% dextrose was mixed with a solution of 5% human albumin and decafluorobutane gas was added to the mixture. Each patient received a bolus injection of PESDA (3 ml) into a peripheral vein, followed by a 10-ml saline flush. As observed in Figures2 and 3, the CEUS study with the microbubble contrast agent was combined with conventional color and power color Doppler US to increase the intensity of the flow signal and to facilitate the characterization of residual flow in the ICA 11,13, although this aspect was not included in our statistical analysis. The images were analyzed with a focus on flow in the interior of the ICA. All examinations were digitally recorded (in AVI, JPEG and MPEG formats) for subsequent analysis.
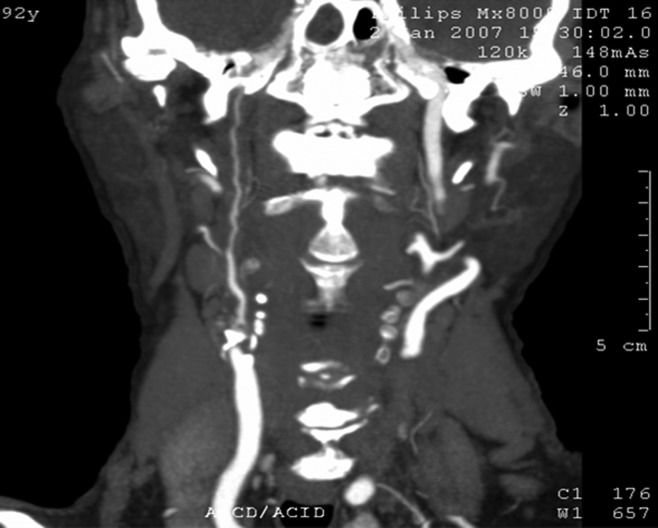
The CTA, as illustrated in Figure4, was performed with a multidetector helical CT scanner (MX 8000; Philips Medical Systems), in slices ≤0.6 mm, at a reconstruction interval of 0.5 mm. Slices were acquired from top to bottom to minimize venous contamination after the injection of non-ionic iodinated contrast, which was administered to the patients in a dose of 2.5 ml/kg at a flow rate of 4 ml/s to a maximum of 100 ml. To guarantee uniform and adequate flow, the CTA contrast agent was administered with an injection pump and all examinations were supervised by a radiologist experienced in bolus tracking. During the contrast injection, we acquired short, fixed tomographic slices of the carotid bodies showing a rising curve based on the Hounsfield (HU) units (≥150 HU). The examinations were post-processed on a CT workstation (Brilliance; Philips Medical Systems) and all examinations were double-read by the two radiologist evaluators. After the examinations were finished, the results were compared to perform the tests described below.
StatisticsDiagnostic measurements were evaluated to calculate the sensitivity, specificity, positive predictive value, negative predictive value and accuracy. For each value, we calculated the 95% confidence interval (95% CI) using Confidence Interval Analysis software, version 2.1.2 (http://www.som.soton.ac.uk/cia/). McNemar's chi-square test was used to compare the proportion of positive results between two types of examinations. Statistical analysis was performed with the Statistical Package for the Social Sciences, version 16.0 for Windows (SPSS Inc., Chicago, IL, USA). Values of p<0.05 were considered statistically significant.
RESULTSWe included 72 patients examined at the HC-FMUSP between June 2006 and June 2012. The mean age ± standard deviation of the patients was 67±11 years (range, 41-96 years). Of the 72 patients, 57 (79.2%) were male. Previous examinations with Doppler US had identified right ICA occlusion in 31 patients, left ICA occlusion in 41 and bilateral ICA occlusion in 6. Therefore, we evaluated a total of 78 carotids.
Comparing the Doppler US results with those of the CTA (Table1), we found that the use of the former resulted in a false-positive diagnosis of ICA occlusion in 21 of the 78 evaluated carotids (26.9%; 95% CI: 17.5-38.2%; p<0.001), a finding that is in accordance with previous reports 9,10. Therefore, there was concordance between the two methods in only 57 of the 78 carotids (73.1%; 95% CI: 61.8-82.5%). The difference between CEUS and Doppler US was also significant (p<0.001). However, comparing the CTA results with the CEUS results (Table2), we found no significant difference between the two methods, with the latter classifying 59 (77.5%) of the 78 carotids evaluated as occluded (p<0.5).
Distribution of the 78 internal carotid arteries classified as occluded by Doppler ultrasound (without contrast), according to the computed tomography angiography results.
| Computed tomography angiography | ||||
|---|---|---|---|---|
| Doppler ultrasound (no contrast) | Occlusion | No occlusion | Total | |
| (n) | (n) | |||
| Occlusion (n) | 57 | 21 | 78 | |
| No occlusion (n) | - | - | - | |
| Total | 57 | 21 | 78 |
Comparison between contrast-enhanced ultrasound and computed tomography angiography in the evaluation of the 78 internal carotid arteries classified as occluded by Doppler ultrasound (without contrast).
| Computed tomography angiography | ||||
|---|---|---|---|---|
| Contrast-enhanced ultrasound | Occlusion | No occlusion | Total | |
| (n) | (n) | |||
| Occlusion (n) | 57 | 2 | 59 | |
| No occlusion (n) | - | 19 | 19 | |
| Total | 57 | 21 | 78 |
Therefore, the false-positive rate for CEUS was 2.6% (2/78) and there were no false negatives. We calculated the sensitivity of CEUS to be 100% (95% CI: 95.4-100%), whereas we found the specificity to be 90.5% (95% CI: 74.9-96.8%); the positive predictive value was 96.6%, the negative predictive value was 100% and the accuracy was 97.4% (95% CI: 91.0-99.7%).
DISCUSSIONInformation about the residual lumen in symptomatic patients suspected of ICA occlusion facilitates decisions regarding the ideal treatment, be it clinical or surgical 2, thereby improving the prognosis and preventing neurologic symptoms along with the cessation of embolic events. Many authors agree that Doppler US can lead to a considerable number of false diagnoses of occlusion 4,5,8,9,20,21. The imaging methods currently used in the diagnosis of ICA occlusion—DSA, CTA and magnetic resonance imaging (MRI)—are undoubtedly accurate 4, and DSA has typically been considered as the gold standard 3,22,23. In similar studies 6,9,12,13,19, DSA has been used as the gold standard, or the reliability of CEUS has been compared with that of MRI. However, CTA has been shown to have excellent sensitivity and specificity for the diagnosis of ICA occlusion 4,5, showing a rate of 100% for stenosis near occlusion and 100% concordance with DSA 4, with the added advantage of being a less invasive method. Because of those considerations, together with its superiority over MRI, CTA has been accepted as the new gold standard 4,5. This led us to choose CTA to test the power of the CEUS approach. As previously stated, the use of microbubble contrast agents substantially increases the intensity of the ultrasound blood flow signals 15 and CEUS has been identified as preferable to Doppler US in various situations, even in cases of suspected ICA occlusion 6,9,11,12,14. Our study has confirmed that CEUS with a second-generation contrast agent is reliable in the diagnosis of ICA occlusion, being similar to CTA in terms of its sensitivity, specificity and accuracy, increasing the already remarkable accuracy of Doppler US in cases of pseudo-occlusion of the ICA.
Our findings corroborate those of other authors, who also found CEUS to be a reliable method that is superior to Doppler US in distinguishing between occlusion and pseudo-occlusion of the ICA 6,9,11,12,14, although the authors of one such study found no significant difference between the two methods 13. However, those studies involved patient samples that were smaller than the sample evaluated in the present study and the latter study involved the use of a first-generation contrast agent, as have other such studies 6,9,12,19,25.
Some of those studies also included patients with known pseudo-occlusion of the ICA 6,13; our study did not.
One interesting aspect of our study was that, unlike the authors of some other studies 11,13, we used color and power Doppler to increase the intensity of the flow signal in CEUS, thus facilitating and speeding up the detection of slow flow in the residual lumen. Although we believe that to be advantageous, we found no subjective difference between the accuracy of CEUS alone and that of CEUS associated with color and power Doppler. It is notable that this aspect was not included in our statistical analysis or in the final results of our study. We also found that CEUS was more reliable that Doppler US, even when the latter was used in combination with power Doppler. In contrast, Furst et al. 19 found no significant difference in the accuracy between power Doppler and CEUS and concluded that CEUS produces a better result only when compared with Doppler US alone. However, it should be kept in mind that power Doppler has a high mechanical index, which can be deleterious to the patient; thus, extra care is highly recommended. As for ultrasound with and without contrast agents, the use of a convex array transducer (4.0-8.0 MHz) was highly relevant to our study; this made it easier to evaluate the ICA, which was clearly visualized even in its most distal segments, allowing for the diagnosis of pseudo-occlusions that would definitely not have been possible using only a linear transducer.
Although it was not the focus of our study, we noticed that CEUS is also useful in evaluating the appearance of the ICA even in its most distal segments, allowing for the assessment of poststenotic dilatation and of possible sequential stenoses, as well as making it possible to determine whether the vessel is a candidate for endarterectomy 9. As previously demonstrated 6,12,26, CEUS facilitates the determination of flow in highly calcified vessels and provides a brighter view of unstable plaque surfaces, making it easier to diagnose ulcerations. In cases of pseudo-occlusion of the ICA, CEUS can better describe the shape of the residual lumen, as we observed in the present study, in which some of the residual lumina presented the “candle flame” shape 2. That residual lumen shape facilitates the placement of the catheter to thread the guide-wire in an endovascular procedure for the clearance of obstruction 2. Therefore, we can infer that CEUS facilitates therapeutic planning by allowing not only an accurate differential diagnosis between occlusion and pseudo-occlusion of the ICA but also a proper evaluation of the morphology of the residual lumen. This observation could become a topic for further studies.
Recent multicenter studies evaluating large patient samples have clearly demonstrated that there is no additional risk associated with ultrasound studies involving microbubble contrast agents 27,28. Although there have been reports of pain or paresthesia at the puncture point, lumbar pain, and (in rare cases) allergic reactions, as well as damage to microcirculation 29, the patients in our study showed no significant adverse reactions to the microbubble contrast medium, exhibiting the same good tolerance reported in the aforementioned studies 27,28. To reduce the bioeffects of the contrast agent, we used a low mechanical index (≤0.4), low-frequency transducers and short acoustic exposure times, as recommended by Barnett et al. 29. In the present study, none of the 72 patients who underwent CTA had any adverse reactions to the iodinated contrast media, indicating good tolerance to the contrast medium; this is also in agreement with the findings of other authors 30,31.
Because of recent advances in the sensitivity of Doppler US equipment, the vast majority of patients with ICA stenosis, regardless of the degree, can be properly diagnosed. The use of CEUS has been reserved for symptomatic patients in whom Doppler US raises the suspicion of occlusion. Although correlation with the surgical procedure would be desirable, only a few of our patients underwent surgery after being diagnosed with pseudo-occlusion. One unfavorable aspect of CEUS in relation to multi-planar examinations is that it limits the evaluation of the intracranial part of the ICA and of the vessels leading to it (the aorta, the brachiocephalic artery and the common carotid artery).
To the best of our knowledge, to date, this study involved the largest patient sample in which CEUS with a second-generation contrast agent has been evaluated as a means of distinguishing between occlusion and pseudo-occlusion of the cervical ICA.
In that regard, we concluded that CEUS is superior to conventional Doppler US and is similar to CTA. Taken together, our data suggest that CEUS can now supplant CTA as the gold standard for the diagnosis of ICA occlusion.
Our findings suggest that symptomatic patients in whom Doppler US indicates ICA occlusion should undergo CEUS to confirm the diagnosis rather than undergoing invasive examinations, which are more costly and more likely to provoke adverse reactions. In addition, CEUS can be an alternative for patients with kidney problems who cannot be screened with either the iodinated contrast media used in CTA and DSA or the gadolinium chelate used in MRI angiography. Furthermore, in patients with a recent pattern of ipsilateral transient ischemic attack, the use of CEUS could facilitate decisions regarding treatments such as endarterectomy or angioplasty with stent placement 2.
Our findings are quite encouraging and corroborate with data in the literature regarding the accuracy of CEUS in distinguishing between occlusion and pseudo-occlusion of the ICA. However, it must be kept in mind that ultrasound is an operator-dependent method and should therefore be performed by experienced physicians or sonographers with the use of appropriate equipment.
To conclude, CEUS with a second-generation contrast agent is another alternative method by which radiologists and vascular surgeons can accurately confirm or exclude a diagnosis of ICA occlusion. That information could then facilitate decisions regarding the ideal treatment.
AUTHOR CONTRIBUTIONSVentura CA, da Silva ES, Cerri GG, Chammas MC, Puech-Leão P, Tachibana A, designed and performed the research, contributed to the new analytical tools, analyzed the data and wrote the paper.
No potential conflict of interest was reported.