Despite evidence suggesting that Doppler ultrasonography can help to differentiate between benign and malignant breast lesions, it is rarely applied in clinical practice. The aim of this study was to determine whether certain vascular features of breast masses observed by duplex Doppler and color Doppler ultrasonography (before and/or after microbubble contrast injection) add information to the gray-scale analysis and support the Breast Imaging-Reporting and Data System (BI-RADS) classification.
METHODS:Seventy solid lesions were prospectively evaluated with gray-scale ultrasonography, color Doppler ultrasonography, and contrast-enhanced ultrasonography. The morphological analysis and lesion vascularity were correlated with the histological results.
RESULTS:Percutaneous core biopsies revealed that 25/70 (17.5%) lesions were malignant, while 45 were benign. Hypervascular lesions with tortuous and central vessels, a resistive index (RI)≥0.73 before contrast injection, and an RI≥0.75 after contrast injection were significantly predictive of malignancy (p<0.001).
CONCLUSION:The combination of gray-scale ultrasonography data with unenhanced or enhanced duplex Doppler and color Doppler US data can provide diagnostically useful information. These techniques can be easily implemented because Doppler devices are already present in most health centers.
Although breast sonograms are not generally used in diagnostic screening for breast cancer, they are a standard procedure for examining dense breasts in women at elevated risk for breast cancer (1,2)and are used to differentiate between benign and malignant masses (3). In 2003, the American College of Radiology published the Breast Imaging-Reporting and Data System (BI-RADS®) lexicon for ultrasonography (US), in which they provided a unified language and a standardized classification system (4). The descriptors used are mainly related to lesion morphology. There are also descriptors related to lesion vascularity; however, these descriptors are simplistic and add little information (i.e., the presence or absence; and localization within the lesion, adjacent to the lesion, or in surrounding tissue).
Malignant tumors commonly produce pro-angiogenic factors that stimulate the growth of new vessels. New vessels differ from native ones in that the new vessels are irregular, tortuous, and of variable caliber, and they form reticular networks with arteriovenous shunts and dichotomous branching (5). Previous reports have indicated that duplex Doppler US (color Doppler associated with pulsed/spectrum analysis) can be used to demonstrate these typical features of neovascularization in the vicinity of malignant lesions (6-8). Contrast-enhanced US (CEUS) is an important diagnostic tool because CEUS can reveal additional information about lesion vascularity. Furthermore, CEUS does not expose patients to ionizing radiation (such as mammography) and is not nephrotoxic, such as magnetic resonance imaging (MRI) contrast agents (9).
Contrast breast US is a relatively new modality, and its applications are still being evaluated. Preliminary studies involving “first-generation” contrast media and color Doppler have yielded encouraging results (10-12). Subsequent studies performed with “second-generation” contrast media (with more stable and durable microbubbles) and harmonic pulse inversion have enabled qualitative and quantitative analyses of contrast-enhanced images, which provide further information about vascular morphologic patterns and distribution features (13-15). Despite these advances, there is still no consensus regarding the use of contrast US for evaluating breast masses.
This study is the first in Brazil to address the clinical applicability of unenhanced and enhanced duplex Doppler in evaluating breast lesions. The aim of the present work was to assess how well gray-scale morphological analysis and lesion vascularity data correlate with the histopathological findings, the gold standard in lesion diagnosis.
MATERIALS AND METHODSPatientsThis study was conducted in the US service department at Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo in São Paulo, Brazil between March 2007 and January 2008. Breast Diagnostics Center patients who had previously been diagnosed with breast lesions (category 4 or 5) and had a clinically or radiologically indicated percutaneous breast biopsy were recruited.
The initial group of samples included 93 breast masses from 73 patients. The patients with atypical pathology results and who did not return for excisional surgery (n = 9) were excluded because an analysis of only a few fragments may not be representative of the entire lesion. The final analysis included 70 solid breast masses in 64 women who were subjected to percutaneous biopsy via core biopsy. These 64 patients ranged in age from 18 to 78 years (median, 49.12 years). All patients provided full informed consent to participate in the study, which was approved by our institutional review board (the Ethics Committee of Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo) on November 23, 2006 (protocol 1021/06).
Study designAll exams were performed by two radiologists, each with more than 10 years of experience with breast US (D.S. and M.C.C.). The US examinations were performed with a TOSHIBA-APLIO XG machine (2007 model year, Tokyo, Japan) equipped with a 5– to 14-MHz linear transducer. Static and dynamic images obtained during the examinations were archived electronically.
Pre-contrast examinationAlthough the masses had already been classified as BI-RADS 4 or 5 at the Diagnosis Center of Breast Diseases in the Hospital das Clínicas, all masses were reevaluated and reclassified, disregarding the prior clinical findings. The lesions presenting typically benign features were categorized as probably benign (BI-RADS 3), even if they were palpable (30 lesions); lesions with 1 suspicious malignancy criterion (or more) were classified as BI-RADS 4 or 5.
Following a gray-scale evaluation, each patient was assessed with color Doppler US (CDUS). The CDUS settings were optimized to detect low-velocity or low-volume blood flow. The color box was adjusted to include the lesion and a margin of normal breast tissue. The color gain was increased until background noise appeared and then reduced until the noise was suppressed and small vessels could be detected. The examinations were performed with minimal probe pressure to prevent vessel collapse.
The lesions were evaluated qualitatively according the criteria described by Moon et al. (12), which are summarized in Table1. We also recorded whether penetrating vessels, which are highly indicative of malignancy, were present, as described by Raza and Baum (7). The lesions were classified as probably benign (avascular or hypovascular lesions with regular and peripheral vessels, Figures1 and 2), probably malignant (hypervascular lesions with irregular vessels and a central distribution, with or without an observable penetrating artery, Figure3), or suspicious (any other pattern variant, Figure4).
Methods and descriptors used to classify lesions.
| Method | Classification | Descriptors | |
|---|---|---|---|
| PRECONTRAST ANALYSIS | |||
| Gray Scale | BI-RADS | BI-RADS Descriptors | BI-RADS 3 |
| Not BI-RADS 3 | |||
| CDUS | Qualitative | Number of Arteries | Avascular (None Detected) |
| Hypovascular (1-2 Arteries) | |||
| Hypervascular (3 or More Arteries) | |||
| Distribution of Arteries | Regular | ||
| Irregular | |||
| Morphology of Arteries | Peripheral | ||
| Central | |||
| Penetrating Artery | |||
| Quantitative | Spectral Analysis | RI | |
| POS CONTRAST ANALYSIS | |||
| PIH + Low MI | Kinectic Analysis | Wash-In | |
| Wash-Out | |||
| Amount of Enhancement | Absent | ||
| Minimal | |||
| Moderate | |||
| Intense | |||
| E-CDUS | Qualitative | Number | Avascular |
| Hypovascular | |||
| Hypervascular | |||
| Distribution | Regular | ||
| Irregular | |||
| Morphology | Peripheral | ||
| Central | |||
| Penetrating Artery | |||
| Quantitative | Spectral Analysis | RI |
An invasive ductal carcinoma recurrence in the left breast of a 63-year-old woman. A) A gray-scale image showing an irregular, spiculated mass with an echogenic halo, architectural distortion, acoustic shadowing, and straightening of Cooper's ligament (BI-RADS 5). B) A color Doppler image showing an avascular lesion. C) After contrast injection, there was minimal enhancement. D) An enhanced color Doppler image showing an avascular lesion.
A fibroadenoma found in the left breast of a 47-year-old woman. A) Gray-scale image showing an irregular, microlobulated mass (BI-RADS 4). B) Color Doppler image showing a peripheral regular artery. C) After contrast injection, we observed moderate peripheral enhancement. D) An enhanced color Doppler image showing the same aspect as that shown for the non-enhanced color Doppler image in B. No additional arteries were observed. Power Doppler imaging was used only to produce clearer images for illustration purposes.
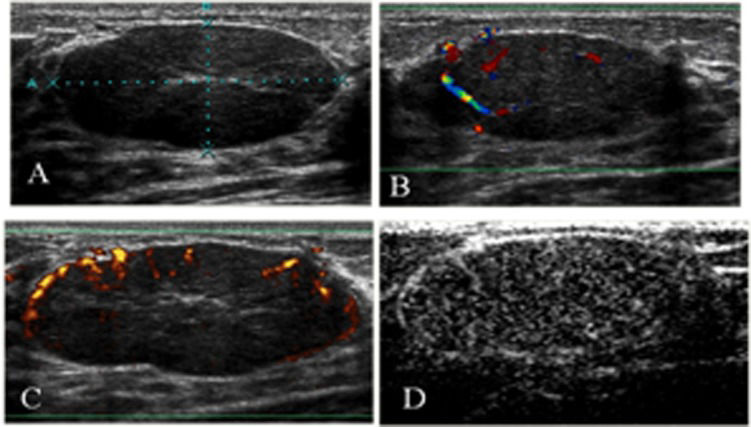
An invasive ductal carcinoma in the right breast of a 71-year-old woman. A) A gray-scale image showing an irregular, microlobulated mass with an echogenic halo and acoustic shadowing (BI-RADS not 3). B) A color Doppler image showing a hypervascular lesion with central and irregular arteries. C) After contrast injection, there was intense enhancement. D) An enhanced color Doppler image showed the same pattern as the unenhanced CDUS. Power Doppler imaging was used only to produce clearer images for illustration purposes.
Juvenile fibroadenoma in the left breast of an 18-year-old woman. A) A gray-scale image showing an oval, circumscribed mass (BI-RADS 3). B) A color Doppler image showing a hypervascular lesion with central and regular arteries. C) An enhanced color Doppler image showed the same pattern as the unenhanced CDUS. Power Doppler imaging was used only to produce clearer images for illustration purposes. D) After contrast injection, there was intense enhancement.
We also performed a quantitative study using spectral Doppler US. The resistive index (RI) was measured for all vessels around hypovascular lesions. For the hypervascular lesions, measurements were taken for three or four different vessels, and the data from the largest vessel were recorded. This criterion was used in isolation and was not considered in the final assessments.
Post-contrast examinationTwo contrast agents were used. A commercially available agent was used with 34 patients (Definity®, Bristol-Myers Squibb, City, MA, USA), and another agent manufactured in our hospital was used with 30 patients (perfluorocarbon-exposed sonicated albumin [PESDA]). The in-house PESDA was prepared as described by Porter at al. (16). None of the patients experienced an adverse reaction.
After catheterizing a peripheral vein, a bolus of contrast medium (3 mL of PESDA or 0.01 mL/kg of Definity®) was injected into the antecubital vessel, followed by 10 mL of saline solution. Before injecting the contrast medium, the display parameters were changed for a contrast exam (pulse-inversion harmonica and mechanical index ≤0.17). As soon as the contrast agent bolus was injected, the time button was activated.
The wash-in time (the delay until reaching the maximum intensity peak), wash-out time (the delay until the maximum intensity inside the mass declined to the baseline), and the amount of enhancement after the contrast agent injection were recorded. Enhancement was classified into four categories: absent (no change in mass echogenicity), minimal (small or equivocal enhancement), moderate (partial enhancement), or intense (maximal/robust enhancement). This classification was performed in a subjective manner based on the observed variation in enhancement intensity in the lesion after the contrast agent was injected because an objective, validated quantitative method for this evaluation type was not available (Table1).
After the wash-out period, we re-analyzed the number of vessels and their characteristics using CDUS after contrast injection and classified the findings based on the parameters described above. We also obtained new vessel RI measurements after the contrast media injection (Table1).
HistopathologyAfter the US analysis, all patients, including those whose masses were graded as BI-RADS 3, returned to the Diagnosis Center of Breast Diseases for a percutaneous breast biopsy with a 14-gauge automated BARD® gun and needle (Bard Radiology, Covington, GA). At least six fragments were removed from each nodule. The fragments were fixed in paraffin and histologically analyzed. The final anatomopathological findings were added to the other findings and subjected to statistical analysis.
Statistical analysisThe statistical analysis was performed using the chi-squared test or the independent t-test (when the former could not be applied). In addition to these tests, receiver operating characteristic (ROC) curves were developed and logistical regression analyses of the data were performed.
RESULTSPre-contrast examinationA total of 70 breast masses from 64 patients were subjected to US analysis and subsequent core biopsies for histopathological correlation. The benign lesions were most commonly diagnosed as fibroadenomas (28/45), stromal fibrosis (6/45), or ductal hyperplasia without atypia (3/45). Approximately 100% of the malignant lesions were diagnosed as invasive ductal carcinomas (23/25), except for one anaplastic neoplasia and one lymphoma. The findings of the final lesion assessments with gray-scale US, unenhanced CDUS, and enhanced CDUS are reported in Table2.
Final breast lesion assessment using gray-scale US, unenhanced CDUS, and enhanced CDUS.
| Final diagnosis | Gray-Scale US | Unenhanced CDUS | Enhanced CDUS | |||||
|---|---|---|---|---|---|---|---|---|
| B3 | Not B3 | PB* | S** | PM*** | PB* | S** | PM*** | |
| Benign | 30 (100%) | 15 (37.5%) | 26 (89.6%) | 14 (66.7%) | 5 (25%) | 21 (91.3%) | 18 (81.8%) | 6 (24%) |
| Malignant | 0 (0%) | 25 (62.5%) | 3 (10.3%) | 7 (33.3%) | 15 (75%) | 2 (8.7%) | 4 (18.2%) | 19 (76%) |
| Total | 30 | 40 | 29 | 21 | 20 | 23 | 22 | 25 |
| Statistical Analysis | ||||||||
| Sensitivity | 100% | 88% | 92% | |||||
| Specificity | 66.6% | 57.7% | 46.6% | |||||
| PPV | 62.5% | 53.6% | 48% | |||||
| NPC | 100% | 89.9% | 91% |
*PB: probably benign; **S: suspicious; ***PM: probably malignant.
The following BI-RADS descriptors were highly predictive of malignancy (p<0.001): vertical orientation (80%), no circumscribed margins (64%), Cooper's ligament changes (100%), ductal changes (78.9%), and skin retraction or irregularity (87%), along with the presence of an echogenic halo (94.4%) or architectural distortion (100%).
Our CDUS analysis revealed that the majority of the malignant lesions were characterized by central vascularity (91.6%), with hypervascularity (68%), a penetrating artery (66.6%), and tortuous vessels (75%). The benign lesions were predominantly avascular or hypovascular (60%) and usually had regular vessels (83.3%). Of the vascular benign masses, only 16.6% had a penetrant artery.
According to the ROC curve, the best RI cut-off value for unenhanced spectral Doppler US (highest sensitivity and specificity) was 0.73; at this value, 76% sensitivity and 71% specificity were achieved. This RI cut-off value allowed significant discrimination between the benign and malignant lesions (p<0.001). The 25 analyzed malignant lesions had a mean RI value of 0.82±0.16 (range, 0.63–1.46), with 13 (80%) of the malignant lesions having an RI value ≥0.73 (p<0.001). The mean RI values of the 45 analyzed benign lesions was 0.48±0.37 (range, 0.46–1.29), with 14 (31.1%) of the benign lesions having an RI value ≥0.73 (p<0.001).
Post-contrast examinationAfter the contrast agent injection, kinetic analyses were used to reveal the enhancement degree, wash-in time, and wash-out time, with the aim of determining whether these parameters provided useful information in classifying the lesions as benign or malignant. Most of the malignant lesions presented moderate (14/25) or intense (7/25) enhancement. No enhancement was observed in one carcinoma and one lymphoma; and minimal enhancement was observed in two carcinomas. For the benign lesions, there was no enhancement in eight cases, minimal enhancement in 11 cases, moderate enhancement in 17 cases, and intense enhancement in nine cases (including five fibroadenomas).
The mean wash-in time for the malignant lesions was 27±11.3 s (range, 14–48 s) and 26.4±10.7 s (range, 14–45 s) for the benign lesions. The mean wash-out time for the malignant lesions was 56.9±32.3 s (range, 19–180 s) and 56.6±22.9 s (range, 33–106 s) for the benign lesions. The ROC curve analysis revealed that the wash-in time and wash-out time parameters did not effectively discriminate between the benign and malignant lesions.
The results of our qualitative enhanced CDUS analysis, following the same criteria used for unenhanced CDUS, are reported in Table2. The vast majority (24/25, 96.0%) of the malignant lesions was hypervascular. Of the 24 hypervascular malignant lesions, 22 (91.6%) had a central distribution, 17 (70.8%) had a penetrating artery, and 20 (83.3%) had tortuous vessels. Approximately half (22/45; 48%) of the benign lesions were avascular or hypovascular. Among the 34 benign lesions that had at least one vessel, a peripheral vascular pattern was observed in 13 (38.2%) lesions, and a central pattern was observed in 21 (61.7%) lesions. Most of the benign lesions with at least one vessel (28/34; 82.3%) had regular vascular morphology.
The best RI cut-off value, according to the ROC curve (highest sensitivity and specificity) in the post-contrast study, was 0.75. With this RI value, 72% sensitivity and 67% specificity were achieved. This value yielded a significant distinction between the benign and malignant cases (p<0.001). The post-contrast quantitative analysis showed that the malignant lesions had a mean RI value of 0.82±0.16 (range, 0.63–1.46), with 19 of the malignant lesions (76%) presenting an RI≥0.75 (p = 0.001). Meanwhile, the benign lesions had a mean RI value of 0.48±0.37 (range, 0.46–1.29), with 17 of the benign lesions (37.8%) having an RI≥0.75 (p = 0.001).
DISCUSSIONIn the present study, BI-RADS classification using gray-scale US examinations showed high sensitivity for detecting malignant lesions but lacked in specificity, producing a large number of false positives. This result pattern corroborates prior BI-RADS classification studies (2,17). The 15 false positives obtained in our study group were mostly fibroadenomas.
Although the BI-RADS lexicon has descriptors concerning mass vascularity, the descriptors are not, in our view, distinctively characteristic of benign versus malignant lesions; nor are they particularly useful for describing, classifying, or managing breast nodules. However, malignant tumors do display characteristic features in duplex Doppler images, such as hypervascularity, vessel penetration, irregular vessels, central vessel distribution, and branching and disordered vessel morphology (6-12). In our study, we found that hypervascularity, tortuous arteries, vessel penetration, and a central vessel distribution pattern were significantly associated with malignancy. Our benignity and malignancy results using duplex Doppler imaging correlated well with our histopathology diagnoses, supporting the notion that our qualitative evaluation criteria were efficacious and valid.
Concerning the quantitative analysis, several studies have described higher RI values in malignant lesions relative to benign lesions (because of arteriovenous shunts and the absence of smooth muscle, resulting in high velocity pulsatile tumoral flow). However, there are no standard cut-off RI values to differentiate between malignant and benignant lesions; previously reported cut-off RI values have ranged from 0.7 to 1.0 (18-21). In our study, optimal sensitivity and specificity values were obtained with an RI of 0.73 for unenhanced CDUS and an RI of 0.75 for enhanced CDUS.
Previous studies have revealed diagnostic pitfalls in unenhanced and enhanced CDUS. Specifically, hypervascularized benign, inflamed lesions, Phyllodes tumors, and fibroadenomas may be mistaken for malignancies, whereas hypovascular carcinomas may be missed and falsely considered to be benign (7),. Our findings were consistent with these prior reports.
Our kinetic data (i.e., the wash-in time, wash-out time, and enhancement intensity after contrast injection) did not differ significantly between the benign and malignant lesions, although we did observe that most of the malignant tumors presented with intense enhancement. It may be that the kinetic analysis failed to produce an association because the analysis was performed subjectively because of the absence of objective tools at the time of the study. However, many authors have described the usefulness of microbubble enhancement for US evaluation of breast masses, and the diagnostic accuracy has been reportedly improved by contrast enhancement (10-12).
More recent studies have shown that not only the analysis of kinetic curves (similar to MRI) (13) but also the distribution pattern of contrast within the mass may suggest malignancy (e.g., heterogeneous or peripheral rim-like hyperenhancement with centripetal filling) (14-15) and a poor prognosis (the presence of a perfusion defect and penetrating vessels) (23). One promising line of research is the study of the effectiveness of neoadjuvant chemotherapy using CEUS; Wan et al. have found that tumor blood perfusion changes occur earlier than tumor size changes (24).
Although unenhanced or enhanced CDUS data alone cannot reliably define lesions as malignant or benign, these data can provide supplementary information to conventional US findings. Consistent with prior work by other researchers (6-12), our study demonstrated that hypervascular lesions with penetrating and irregular vessels and a central distribution are related to malignancy. Thus, we believe that these descriptors could improve the BI-RADS classification and, therefore, should be included in the BI-RADS lexicon.
Our study had a noteworthy limitation: namely, the lack of an objective method of analyzing the kinetic patterns. Consequently, the true potential of enhanced breast US cannot be conclusively determined at this time. Despite this limitation, this study demonstrated the importance of the vascular analysis of breast masses, which can be easily performed with unenhanced CDUS.
In conclusion, the present work indicates that the vascular characteristics observed with spectral and color Doppler US are associated with the morphological descriptors in the BI-RADS lexicon, thereby providing new and relevant information for determining the nature of breast lesions. Considering that Doppler functionality is available in virtually all modern US equipment and that this method could be implemented easily without adding significant costs to conventional breast US, we recommend that data obtained by spectral and color Doppler US be incorporated into the routine clinical analysis of breast lesions.
AUTHOR CONTRIBUTIONSStanzani D was the main author and was responsible for the study methods, patient exams, data collection, results, discussion, and conclusion. Chala L and Barros N were responsible for the patient selection and biopsies. Cerri GG was responsible for the text revision. Chammas MC was responsible for the study methods, patient exams, discussion, and conclusion.
No potential conflict of interest was reported.