Carcinoid tumors of Vater’s ampulla are the most common among the extremely rare primary ampullary neuroendocrine tumors, accounting for less than 0.35% of all gastrointestinal carcinoids and are frequently associated with Von Recklinghausen’s disease.1,2
Carcinoid tumors of Vater’s ampulla are difficult to diagnose preoperatively because of their relatively small size and submucosal location.1–4 Consequently, their true nature is usually only discovered after appropriate immuno-cytochemical, histochemical, or ultrastructural studies.5,6
Carcinoid tumors of the minor papilla are also very rare. To the best of our knowledge, only 7 cases have been reported in the literature.6–12 That carcinoid tumors of the major duodenal papilla have been described more frequently than those of the minor papilla is probably because those of the major duodenal papilla often give rise to symptoms (for example jaundice and pain) that demand intensive examination or surgery; while those of the minor papilla are frequently asymptomatic.13 These gastrointestinal tumors appear to be hormonally inactive, and abdominal pain is the main clinical feature.14
Pancreas divisum is the most common congenital variant of the pancreatic anatomy and occurs when the ductal systems of the ventral and dorsal pancreatic ducts fail to fuse.15–17 Because the ducts are not completely united, most of the pancreatic exocrine secretions enter the duodenum via the dorsal duct and minor papilla. Of the 7 cases of carcinoid tumors of the minor papilla reported in the literature, 4 were associated with pancreas divisum.
This report describes a case of an endocrine tumor of the carcinoid type, immunohistochemically a somatostatinoma, which was localized in the minor papilla and associated with pancreas divisum.
CASE REPORTA 57-year-old, white woman presented epigastralgia, steatorrhea, and an 8 kg weight loss, with an onset 1 year previously. Laboratory exams were normal except for the following: (i) presence of anemia (hemoglobin 7.5 g%), (ii) positive test for occult blood in the stool, and (iii) strongly positive (+++/4) test for fecal fat. Colonoscopy did not reveal abnormalities. Gastroduodenoscopy identified an elevated submucosal lesion with an integral mucous membrane, 2 cm in diameter, in the second part of the duodenum; the major duodenal papilla exhibited a normal aspect. Histopathologic exam of the duodenal lesion revealed nonspecific chronic duodenitis. Contrasted radiography of the small intestine revealed a filling defect in the second part of the duodenum, with preservation of the mucosal folds (Figure 1). Magnetic resonance cholangiopancreatography showed a pancreas with reduced dimensions, irregular borders, and dilation of the pancreatic dorsal duct throughout its extent, compatible with pancreas divisum and a chronic inflammatory process; the accessory pancreatic duct originated in the duodenal loop, with the distal portion juxtaposed to the main pancreatic duct (Figure 2). In the second part of the duodenum, there was a solid, very well defined nodular image from which the duct originated. This duct extended into the cephalic area of pancreas and implanted its distal portion in the main pancreatic duct. Endoscopic retrograde cholangiopancreatography revealed a submucosal lesion involving the minor duodenal papilla and for which the ostium was not observed. The major duodenal papilla exhibited a preserved aspect, and administration of contrast medium into its ostium showed a fine and short pancreatic ductal branch, corresponding anatomically to the main pancreatic duct, thereby suggesting pancreas divisum.
Magnetic resonance cholangiopancreatography with exogenous administration of secretin showing both the ventral duct and the dorsal pancreatic duct without connection between them. Santorini’s duct is slightly enlarged. The pancreas presents reduced dimensions and irregular borders compatible with a chronic inflammatory process.
The patient underwent exploratory laparotomy, and duodenotomy was performed. The major duodenal papilla was identified, and presented a preserved aspect. The presence of the submucosal lesion was verified in the second part of the duodenum, being approximately 2.5 cm in diameter and having an integral mucosa (Figure 3). An orifice was identified in the apex of the duodenal lesion, which was catheterized, and contrast medium was introduced. An extensive and tortuous accessory pancreatic duct was observed (Figure 4). Frozen-section biopsy revealed a hyperplastic polyp; however, histopathologic exam in paraffin showed a carcinoid tumor of the duodenum. In a second operation, the patient underwent a partial pancreatoduodenectomy (Whipple’s operation). Macroscopic examination revealed a tumor in the duodenal papilla measuring 2.7 cm in its largest diameter. Histologically, the tumor was characterized as sheets and nests of small cells with regular nuclei and “salt and pepper” chromatin (Figure 5), with perineural infiltration. The immunohistochemical study was positive for the pan-endocrine markers (neuron-specific enolase, synaptophysin, and chromogranin) as well as for somatostatin (Figure 6) and negative for insulin, glucagon, serotonin, cytokeratins AE1/AE3, and carcinoembryonic antigen. Nine lymph nodes were identified and were free from involvement by the tumor. The final diagnosis was a typical carcinoid of duodenal papilla.
Aspect of the submucosal lesion in the second part of the duodenum after duodenotomy, being approximately 2.5 cm in diameter and having an integral mucosa. The dark area corresponds to the frozen-section biopsy site. A catheter was introduced into the orifice close to the apex of the lesion.
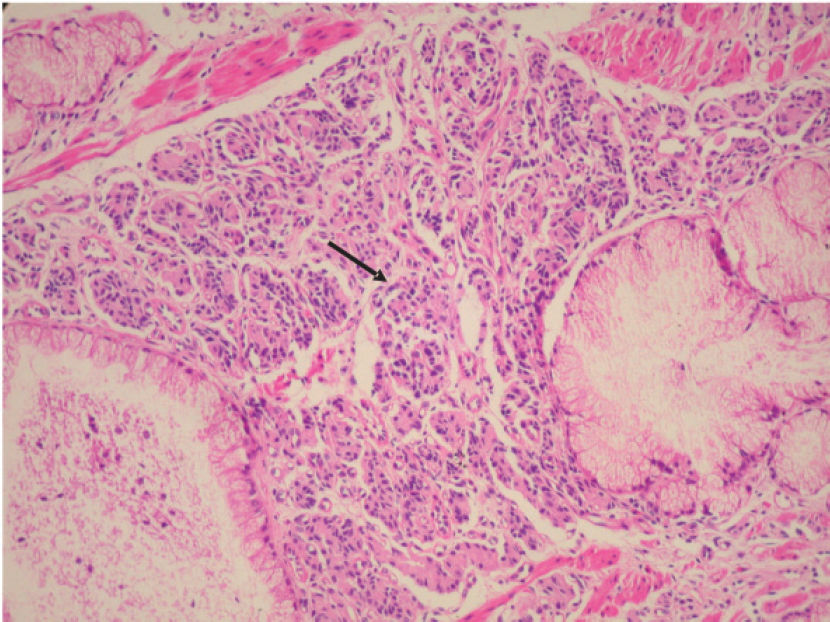
Photomicrograph of somatostatinoma of the minor papilla. The tumor is composed of nests of uniform cells (arrow) forming a trabecular pattern, eosinophilic granular cytoplasm, and the typical organoid arrangement of neuroendocrine cells. The nuclei are small and show fine chromatin (salt-pepper chromatin). (hematoxylin & eosin staining; original magnification × 200).
Somatostatin immunoreactivity in the ampullar lesion displaying uniform intense staining. Tumor cells were visible in this serotonin-containing carcinoid tumor of the minor ampulla (large arrow). Ductal epithelial cells are negative. Medium-to-large cells with pale, granular cytoplasm and round nuclei without mitotic activity can be seen (small arrow). (immunoperoxidase, × 400).
The patient was recovering satisfactorily until postoperative day 21, when she presented pulmonary thromboem-bolism that developed into severe respiratory insufficiency. The patient died due to severe cardiopathy with pulmonary hypertension.
DISCUSSIONOn histologic examination, these neoplasms are arranged in compact nests, ribbons, trabeculae, or in a diffuse, solid pattern. Cytological examination shows neoplasms composed of a uniform population of cells with central round nuclei and granular or “salt and pepper” chromatin, rare-to-no mitotic activity, and a moderate amount of finely granular cytoplasm.2,14 Extrapancreatic somatostatinomas are characterized histologically by the presence of psammoma bodies,13 which result from the intense secretory activity of neoplastic cells, normally located in the glandular spaces. However, psammoma bodies were not observed in this case.
Immunohistochemically, tumor cells express somatostatin in 67%, serotonin, cholecystokinin in 17%, and insulin in 25% of cases. Glucagon and gastrin are generally not expressed by ampullary carcinoid tumors.5,6
Since carcinoids of the duodenal ampulla are rarely accompanied by endocrine symptoms,18–20 it is even more difficult to diagnose them on a clinical basis. In the case reported here, the clinical picture at presentation (epigastralgia, diarrhea, and weight loss) could be attributed to chronic pancreatitis associated with pancreas divisum and aggravated by neoplastic obstruction of the minor duodenal papilla.
Furthermore, a correct preoperative diagnosis is more difficult because biopsies are often negative, as occurred in the present case. Duodenoscopy with endoscopic retrograde cholangiopancreatography may suggest the diagnosis, but extensive and deep biopsies are often required to confirm the histologic nature of the tumor.2–4 Positive tissue diagnosis is optimized when biopsies are taken from the edges of the papillotomy wound or snare biopsies of a protruded papilla are performed.2,3
The clinical and morphologic characteristics of the 8 patients with reported carcinoid tumors of the minor papilla are summarized in Table 1.
Main clinical aspects, morphologic features, treatment modalities, and outcome of cases of carcinoid of the minor duodenal papilla
| Author | Sex | Age | Clinical picture | Pre-operative diagnosis: Pancreas Divisuum | I | Treatment | Size(cm) | Metastasis | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Malone et al6 | M | 46 | Epigastric pain, weight loss, anorexia, steatorrhea | Yes | No | Local resection | 0.5 – 0.75 | No | Alive, 16 months |
| Stömmer et al7 | F | 56 | Jaundice, weight loss | No | Yes | Duodenopancreatectomy | 0.3 | No | No information |
| Lowes et al8 | F | 50 | Abdominal pain, weight loss, diarrhea | Yes | Yes | Duodenopancreatectomy | 1.0 – 2.0 | Lymph nodes | No information |
| Borobia et al9 | F | 46 | Neurofibromatosis, diarrhea, | ||||||
| steatorrhea | Yes | No | Local resection of papilla * | No information | No | Alive, 3 years | |||
| Singh et al10 | F | 35 | Relapsing pancreatitis | Yes | Yes | Sphincteroplasty | 1.0 | No | Alive, 6 months |
| Outtas et al11 | F | 45 | Nodular panniculitis | Yes | Yes | Duodenopancreatectomy | 0.6 | No | Alive, 1 year |
| Wang et al12 | M | 50 | Multiple melenas, | ||||||
| polycythemia vera | Yes | No | Transduodenal resection | 0.9 | No | Alive, 3 years | |||
| Waisberg et al (present case) | F | 57 | Abdominal pain, diarrhea and weight loss | No | Yes | Duodenopancreatectomy | 2.7 | No | Dead, day 21, postoperative complications |
A review by Hatzitheoklitos et al20 shows that 46% of major duodenal ampulla carcinoids greater than 2 cm, 50% of tumors between 1 and 2 cm, and 66% of tumors less than 1 cm in diameter exhibit metastases. These data indicate that carcinoids involving the major duodenal ampulla metastasize in 50% of cases, irrespective of primary tumor size. Thus, tumor size alone cannot clinically determine the extent of the operation.19 This, in addition to the safety of pancreatoduodenectomy when performed in specialized centers, indicates that
Whipple’s operation is the most appropriate treatment for ampullary carcinoids.19
However, local excision with lymph node dissection has been suggested for tumors less than 2 cm in diameter.19 Provided that the main pancreatic duct is not involved, this form of treatment can be expected to achieve good results in terms of being free from operative complications. On the other hand, when the pancreatic duct is involved, the operative morbidity is higher, so some advantages that might be gained from local excision are lost. In such cases and particularly with more extensive local involvement, pancreatoduodenectomy is indicated.20 The prognosis for carcinoid of the minor duodenal papilla following treatment is good, reaching a 5-year survival of 90%;13 nevertheless, a meticulous follow-up after surgical resection is indicated since some carcinoid tumors can metastasize.10