Understanding the changes in chondrogenic gene expression that are involved in the differentiation of human adipose-derived stem cells to chondrogenic cells is important prior to using this approach for cartilage repair. The aims of the study were to characterize human adipose-derived stem cells and to examine chondrogenic gene expression after one, two, and three weeks of induction.
MATERIALS AND METHODS:Human adipose-derived stem cells at passage 4 were evaluated by flow cytometry to examine the expression of surface markers. These adipose-derived stem cells were tested for adipogenic and osteogenic differentiation capacity. Ribonucleic acid was extracted from the cells for quantitative polymerase chain reaction analysis to determine the expression levels of chondrogenic genes after chondrogenic induction.
RESULTS:Human adipose-derived stem cells were strongly positive for the mesenchymal markers CD90, CD73, CD44, CD9, and histocompatibility antigen and successfully differentiated into adipogenic and osteogenic lineages. The human adipose-derived stem cells aggregated and formed a dense matrix after chondrogenic induction. The expression of chondrogenic genes (collagen type II, aggrecan core protein, collagen type XI, COMP, and ELASTIN) was significantly higher after the first week of induction. However, a significantly elevated expression of collagen type X was observed after three weeks of chondrogenic induction.
CONCLUSION:Human adipose-derived stem cells retain stem cell characteristics after expansion in culture to passage 4 and serve as a feasible source of cells for cartilage regeneration. Chondrogenesis in human adipose-derived stem cells was most prominent after one week of chondrogenic induction.
The repair of cartilaginous defects remains a significant clinical challenge, due to the poor spontaneous regeneration of articular cartilage. This poor regeneration is a result of an insufficient blood supply and the sparse number of chondrocytes embedded in the dense extracellular matrix of articular cartilage. Chondrocyte-based therapy for cartilage repair has demonstrated promising clinical outcomes (1). While this procedure is generally successful, obtaining a sufficient number of autologous chondrocytes for the treatment of large cartilage defects is difficult. Moreover, the procedure requires an invasive protocol for harvesting the autologous cartilage from a non-weight-bearing site of the patient's knee cartilage and creates additional damage to normal cartilage. Studies have shown that the donor site for autologous chondrocyte harvesting can experience pathological changes to the articular cartilage in the joint (2). Clearly, an alternative source of cells must be used to avoid the shortcomings of using autologous chondrocytes.
Previous studies have indicated that adult mesenchymal stem cells can be found in human adipose tissue (3). Adipose tissue represents a good candidate tissue for obtaining adult stem cells for regenerative therapy because it is readily available in a large quantity and can be obtained through less invasive procedures. Furthermore, the stromal vascular portion of adipose tissue has been reported to contain up to 2% of cells that are able to differentiate into various cell types, such as osteoblasts, chondrocytes, adipocytes, and neurons, compared with only 0.002% of cells with this capability in bone marrow (4). Flow cytometry analysis has been extensively used to examine the surface immunophenotype of stem cells isolated from humans and other species. Previous studies have shown that human adipose derived stem cells (HADSCs) express a characteristic adhesion molecule (CD9), mesenchymal stem cell markers (CD90, CD44, and CD73), and the histocompatibility antigen HLA-ABC. Meanwhile, hematopoietic antigens (CD31, CD34 and CD45), a stem-cell factor (CD117), and the histocompatibility antigen HLA-DR-DP-DQ have been reported to be antigens that are absent from the surface of HADSCs (3). To assess whether HADSCs retain the immunophenotype of stem cells after expansion in culture, flow cytometry analysis was performed using the panel of antibodies described above. Due to the ease of their collection and their capacity for multipotential differentiation, HADSCs are worthy of consideration as a source of cells for treatment of cartilage lesions. Stem cells can be easily expanded in culture for several passages to obtain a sufficient and homogeneous population of cells before differentiating into cartilage-generating cells (chondrocytes). However, the characteristics and differentiation capacity of serially passaged HADSCs have not yet been reported in detail.
In the developing embryo, all bones of the axial skeleton are formed via cartilage in an intermediate step in the process of endochondral ossification. During this process, mesenchymal cells condense and begin to differentiate along a pathway that ultimately leads to chondrocyte hypertrophy and mineralized tissue. O'Driscoll et al. have proposed a model that includes four stages of chondrogenesis: (a) commitment of the cells to chondrocytes (0-48 hours), (b) cell proliferation (1-10 days), (c) differentiation (7-28 days), and (d) matrix production (10-42 days) (5). Chondrogenesis begins with the aggregation and condensation of loose mesenchymal stem cells. During this early event, the condensing mesenchyme expresses various extracellular matrix (ECM) components, including collagen type II, aggrecan core protein, cartilage oligomeric matrix protein (COMP), and the important transcription factor SRY box 9 (Sox9) (6,7). As the mesenchymal stem cells differentiate into chondrocytes, the cells begin to produce ECM materials rich in aggrecan core protein and COMP.
Chondrogenesis by HADSCs may involve a process similar to that described above. However, to date, the detailed molecular changes during the chondrogenic induction of HADSCs have not been examined, although this information is crucial to determine if a cell-based therapy protocol is useful for repairing cartilage lesions. To understand the molecular events that are involved in the differentiation of uncommitted HADSCs to chondrocytes and the formation of cartilage tissue, we investigated the sequential expression of chondrogenic genes in HADSCs during chondrogenic induction after the first, second, and third week of culture. Knowledge of the rapid changes in chondrogenic gene expression during chondrogenic induction in HADSCs will provide a better understanding of the potential of these stem cells for cartilage repair. Knowledge of genes that are expressed during chondrogenic induction in culture is of fundamental importance for the use of stem cell-based approaches for the treatment of cartilage lesions.
MATERIALS AND METHODSHuman adipose-derived stem cell isolation and cultureThis study was approved by the Universiti Kebangsaan Malaysia Research and Ethical Committee (Project code FF-083-2007). Lipoaspirate was obtained from consented patients (n = 6) after liposuction procedures that were performed at the Subang Jaya Medical Center in Malaysia. The specimens were placed in sterile containers and brought to the Biotechnology Laboratory, Department of Physiology, Faculty of Medicine, Universiti Kebangsaan, Malaysia to be processed within 24 hours. Each specimen was digested with 0.3% collagenase type I (Invitrogen, Carlsbad, CA, USA) in an orbital incubator at 37°C for four hours. Next, the cell suspension was centrifuged, and the cell pellet was washed with phosphate-buffered saline (PBS, Invitrogen) to remove red blood cells and other impurities. After a final centrifugation, the cell pellet was suspended in the control medium Ham's F12:Dulbecco's Modified Eagle Medium (1∶1) supplemented with 10% fetal bovine serum (FBS), 1% antibiotic and antimycotic, 1% glutamax (Invitrogen), and 1% Vitamin C (Sigma, St. Louis, MO, USA). All cultures were maintained in a 5% CO2 incubator at 37°C. The medium was changed every three days. After reaching 70% confluence, the primary culture (P0) was trypsinized using 0.05% trypsin-EDTA (Invitrogen) and passaged at a culture expansion ratio of 1∶4 until passage 4 (P4).
Flow cytometry analysisFlow cytometry was performed on HADSCs at P4. The HADSCs were trypsinized using Accutase (Innovative Cell Technologies, San Diego, CA, USA). The cell suspension was subsequently centrifuged at 1200 rpm for 10 minutes and washed with DPBS containing 0.5% Bovine Serum Album (Sigma). HADSCs were filtered through a 70-µm nylon membrane, and the number of cells was determined by hemocytometer. A total of 2×105 cells were incubated with either fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated antibodies for 30 minutes. The following CD surface markers were tested: CD9/PE, CD34/FITC, CD117/PE, CD31/PE, CD44/FITC, CD45/FITC, CD73/FITC, CD90/FITC, HLA-ABC/FITC and HLA-DR-DP-DQ/FITC (BD Biosciences, NJ, USA). Ten thousand events were acquired for each CD surface marker on a Becton Dickinson FACSCalibur flow cytometer. The data analysis was performed using CELLQuestPro acquisition software (BD Bioscience, Franklin Lakes, NJ, USA).
Adipogenic induction of human adipose-derived stem cells (HADSCs)Isolated HADSCs were expanded in culture until passage 4 (P4) in an equal volume mix of Ham's F-12 medium and Dulbecco's Modified Eagle Medium supplemented with 10% FBS (control medium). The HADSCs were induced in an adipogenic induction medium that contained 200 µM indomethacin, 10 µM insulin, 0.5 mM 3-isobutyl-1-methylxanthine, and 1 µM dexamethasone (Sigma) at a seeding density of 400 cells/mm2. Media were replaced every three days, and the cells were maintained in culture for up to three weeks. After three weeks, the cultures were fixed in 10% formalin, and adipogenic differentiation was determined by Oil Red O staining.
Osteogenic induction of human adipose-derived stem cells (HADSCs)HADSCs at passage 4 (P4) were induced in an osteogenic induction medium that contained 100 nM dexamethasone, 10 mM β-glycerophosphate, and 5 µg/ml ascorbate-2-phosphate (Sigma) at a seeding density of 200 cells/mm2. The cultures were fed with the osteogenic medium every three days for up to three weeks. After three weeks, the cultures were fixed in 10% formalin, and osteogenic differentiation was determined by Alizarin Red staining.
Chondrogenic induction of human adipose-derived stem cells (HADSCs)HADSCs at P4 were cultured in T25 flasks at a cell density of 9.6×104 cells/cm2 in either the chondrogenic induction medium or the control medium. The chondrogenic induction medium contained F12:DMEM (1:1) supplemented with 1% FBS, 1% antibiotic and antimycotic, 1% glutamax, 1% vitamin c, 1% insulin-transferrin-selenium-X (ITS), 50 ng/mL insulin-like growth factor 1 (IGF-1), 50 µg/mL ascorbate-2-phosphate, 100 nM dexamethasone, 40 µg/ml L-proline, and 5 ng/mL transforming growth factor-β3 (TGF-β3) (Sigma). The morphological features of cultured cells were monitored every day using an inverted light microscope (Olympus, Shinjuku-ku, Tokyo, Japan). HADSCs were harvested after the first, second and third week of culture for the quantification of sequential chondrogenic gene expression.
Total RNA isolation and quantitative PCR analysisTotal RNA was extracted from cultured HADSCs after the first, second, and third week of culture in either the control medium or the chondrogenic induction medium using TRI Reagent (Molecular Research Center, Cincinnati, OH) according to the manufacturer's protocol. The HADSCs were homogenized in TRI reagent and centrifuged at 12,000 x g for 15 minutes at 4°C to separate the cell debris. Total RNA was precipitated with 10 µL Polyacryl Carrier (Molecular Research Center). The total RNA pellet was then washed with 75% ethanol, dissolved in RNase- and DNase-free water (Invitrogen) and stored at -80°C until use. Complementary DNA (cDNA) was synthesized from total RNA using Superscript III reverse transcriptase (Invitrogen). The reaction mix and protocol were performed following the manufacturer's recommendations. The reaction cycle was 10 min at 23°C, 60 min at 42°C, and 10 min at 94°C. To quantify the expression level of chondrogenic genes after the first, second, and third week of culture in the chondrogenic induction medium or the control medium, quantitative polymerase chain reaction (qPCR) was performed using cDNA as the template. The genes of interest were collagen type II, aggrecan core protein, SOX9, COMP, ELASTIN, collagen type XI, collagen type I (dedifferentiation marker), and collagen type X (hypertrophy marker). The specific primer sequences were designed using Primer 3 software (http://frodo.wi.mit.edu/primer3/) based on the published GenBank database sequences (Table 1). QPCR was performed in a Bio-Rad iCycler (Bio-Rad, Hercules, CA, USA), and the data were analyzed using the Bio-Rad iCycler software. Each qPCR mixture consisted of iQ SYBR Supermix (Bio-Rad), forward and reverse primers (5 µM of each) and 1 µl of cDNA template. The following PCR conditions were used: cycle 1: 95°C for 3 minutes (1X) and cycle 2: Step 1, 95°C for 10 second and Step 2, 61°C for 30 second (40X). The PCR cycles were followed by a melting-curve analysis to determine the specificity of the PCR products. Next, the data were normalized to the expression of the housekeeping gene GAPDH. The formula for calculating the relative mRNA expression from the data are as follows:
List of primers used in qPCR for chondrogenic genes (www.ncbi.nlm.nih.gov/nucleotide).
| Chondrogenic Gene | Gene Bank Accession No. | Primer sequences |
|---|---|---|
| Human Collagen Type I, Col I | NM_000088 | F: 5′-agg gct cca acg aga tcg aga-3′R: 5′-tac agg aag cag aca ggg cca-3′ |
| Human Collagen Type II, Col II | NM_001844 | F: 5′-cta tct gga cga agc agc tgg ca-3′R: 5′-atg ggt gca atg tca atg atg g-3′ |
| Human Collagen Type X, Col X | NM_000493 | F: 5′-gct aag ggt gaa agg ggt tc-3′R: 5′-ctc cag gat cac ctt ttg ga-3′ |
| Human Collagen Type XI, Col XI | NM_080681 | F: 5′-agc agg ggg tga gac ctg tg -3′R: 5′-tgc tcc aga gca ggg gta gg-3′ |
| Human Aggrecan Core Protein, ACP | NM_001135 | F: 5′-cac tgt tac cgc cac ttc cc-3′R: 5′-acc agc gga agt ccc ctt cg-3′ |
| Human Sox9, SOX9 | NM_000346 | F: 5′-gcg gag gaa gtc ggt gaa ga-3′R: 5′-ccc tct cgc ttc agg tca gc-3′ |
| Cartilage Oligomeric Protein, COMP | NM_000095 | F: 5′-aac tca ggg cag gag gat gt-3′R: 5′-tgt cct ttt ggt cgt cgt tc-3 |
| ELASTIN | NM_000501 | F: 5′-ggc ctg gag gca aac ctc tt-3′R: 5′-cca cca act cct ggg aca cc-3′ |
| GAPDH | NM_002046 | F: 5′-tcc ctg agc tga acg gga ag-3′R: 5′-gga gga gtg ggt gtc gct gt-3′ |
Relative mRNA expression: 2ΔΔCT
ΔΔCT = CT housekeeping gene – CT gene of interest
CT = Threshold cycle (relative measure of the concentration of the target in the PCR reaction)
Statistical AnalysisThe data are presented as the mean ± the standard error of the mean (SEM). The gene expressions of collagen type II, aggrecan core protein, SOX9, COMP, ELASTIN, collagen type I and collagen type X in the control and chondrogenic induction groups after induction for one, two and three weeks were subjected to one-way analysis of variance with a Tukey post-hoc test and were analyzed using SPSS 15.0 (SPSS Inc., Chicago, IL). A p-value<0.05 was considered to be significant.
RESULTSImmunophenotype of HADSCsHADSCs displayed positive staining for the mesenchymal surface markers CD90, CD73, CD44, CD9, and HLA-ABC (Table 2). HADSCs at passage 4 exhibited high levels of expression of CD90, CD73, CD9 and CD44, which stained more than 90% of the total cell population. More than 99% of HADSCs expressed the histocompatibility antigen molecule HLA-ABC. In contrast, only a small proportion (less than 7%) of HADSCs expressed the hematopoietic markers CD117, CD34, CD31, CD45, and the histocompatibility antigen class II HLA-DR-DP-DQ.
Immunophenotyping of human adipose-derived stem cells (HADSCs) at passage 4. Data are presented as the mean ± standard error of the mean (SEM). n = 6.
| Surface Markers | HADSCs at P4 |
|---|---|
| CD90 | 99.9±0.1 |
| CD9 | 91.2±2.5 |
| CD73 | 99.8±0.1 |
| CD44 | 99.2±0.2 |
| HLA ABC | 99.3±0.4 |
| CD45 | 6.2±0.7 |
| CD117 | 5.8±0.8 |
| CD31 | 1.9±0.3 |
| CD34 | 2.4±0.5 |
| HLA DP, DQ, DR | 3.0±0.3 |
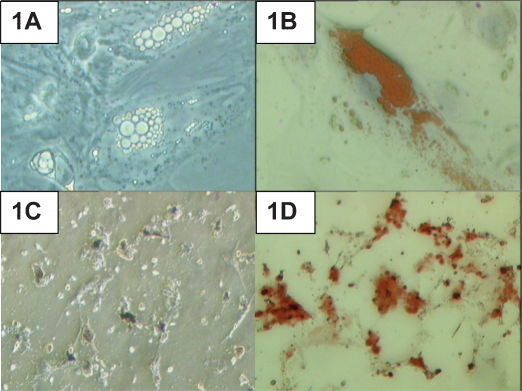
After 21 days of culture in the adipogenic induction medium, lipid droplets had formed in the induced HADSCs (Figure 1A). Positive Oil Red O staining further confirmed the formation of lipid droplets in the culture (Figure 1B). Under osteogenic induction conditions, dark extracellular matrix material was detected after the induction period (Figure 1C). Deposition of calcified matrix by the cells was confirmed by positive staining with Alizarin Red (Figure 1D).
Human adipose-derived stem cells (HADSCs) cultured in adipogenic and osteogenic induction media. (A) Lipid droplet production in HADSCs after 21 days of adipogenic induction. (B) Positive Oil Red O staining of the lipid droplets. (C) Calcium deposition in HADSCs after 21 days of osteogenic induction. (D) Positive Alizarin Red staining. Magnification 400X.
After HADSCs were incubated in the chondrogenic medium for 24 hours, the cells began to move closer to one another and form cell aggregates. This aggregation demonstrated that cellular condensation activity started shortly after chondrogenic induction. The cell aggregates became larger after a week in culture (Figure 2A). After 14 days in the chondrogenic induction medium, most of the cells in the culture were involved in aggregate formation, and empty areas were present in the culture flasks (Figure 2B). On day 21 of chondrogenic induction, the cell aggregates became darker in color, which denoted the formation of dense cell aggregates with increased extracellular matrix production (Figure 2C). HADSCs in the control medium did not form any cell aggregates during the three-week culture period. HADSCs in the control medium continued to grow and form multilayers at the end of the third week of culture (Figure 2D).
Human adipose-derived stem cells (HADSCs) cultured in a chondrogenic induction medium. (A) After one week of chondrogenic induction, HADSCs showed early cell condensation (arrow). (B) Cell aggregates (arrow) became larger in size and started to store matrices during the second week of chondrogenic induction. (C) Cell aggregates (arrow) became dense and dark in color, which denoted increased matrix deposition at the third week of culture in a chondrogenic induction medium. (D) HADSCs grown in a control medium did not show any cell aggregation after three weeks in culture. Magnification 200X.
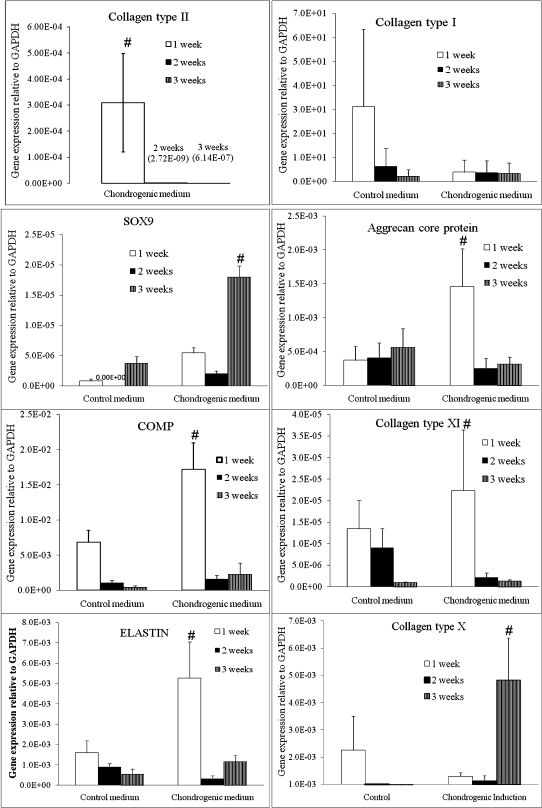
The HADSCs cultured in the chondrogenic induction medium displayed a significantly higher expression of chondrogenic genes (collagen type II, SOX9, aggrecan core protein, COMP, collagen type XI, and ELASTIN) than the control group (Figure 3). Collagen type II mRNA was expressed only in the chondrogenic induction groups. The HADSCs expressed significantly higher levels of collagen type II mRNA after the first week (3.09E-04±1.89E-04) in the chondrogenic induction medium compared with both the second week (2.72E-09±2.72E-09) and the third week (1.28E-07±1.28E-07) of culture. Aggrecan core protein (ACP) was also expressed at the highest level after the first week of chondrogenic induction. The induced cells displayed a fourfold higher ACP expression after the first week (1.46E-03±0.56E-03) compared with the same culture duration of the control group. Subsequent culturing of the cells in the chondrogenic medium caused a reduction in ACP expression to 2.50E-04±1.46E-04 at the second week and 3.13E-04±1.02E-04 at the third week of culture. Similarly, COMP expression was significantly higher in the HADSCs after the first week of culture in the chondrogenic induction medium compared with the other groups. The HADSCs expressed 2.5-fold higher COMP mRNA expression after the first week of culture compared with the control group, 10-fold higher COMP expression after the second week and 8-fold higher COMP expression after the third week. Both ELASTIN and collagen type XI were also expressed at the highest level after the first week of chondrogenic induction compared with the other groups. However, SOX9 expression reached the highest level at the third week of culture in the chondrogenic induction medium. SOX9 expression at the third week was significantly higher than at the first and second week in the chondrogenic induction group and all of the control groups.
Chondrogenic gene expression in human adipose-derived stem cells cultured for one, two, and three weeks in a chondrogenic induction medium compared with a control medium. A significantly higher expression of the following chondrogenic genes was observed in the chondrogenic induction medium compared with the control medium in the first, second, and third weeks of culture: Collagen type II (a), Collagen type X (b), Collagen type XI (c), Aggrecan core protein (d), SOX9 (e), COMP (f) and ELASTIN (g). n = 6, p<0.05.
In contrast, collagen type I (a dedifferentiation marker) was expressed at significantly higher levels at the first week of HADSC culture in the control medium. HADSCs in the chondrogenic induction medium expressed a much lower level of collagen type I expression; this expression was maintained at a steady state throughout the three weeks of culture compared with the control groups.
Finally, HADSCs expressed significantly higher levels of collagen type X (a hypertrophy marker) mRNA expression at the third week of culture in the chondrogenic induction medium compared with the other groups. HADSCs expressed 8.5-fold higher collagen type X mRNA expression at the third week of culture compared with cells cultured in the control medium for the same culture period and 4-fold higher collagen type X expression compared with the first and second week of culture of HADSCs in the chondrogenic medium.
DISCUSSIONThe characterization of any source of stem cells after expansion in culture is crucial for determining if the cultured cells retain the stemness criteria required for cell-based therapy and testing purposes. HADSCs were cultured to passage 4 to obtain sufficient cells for analysis in this study. Standard characterization methods assess the expression of mesenchymal markers and the multilineage differentiation capacity of the stem cells. Based on flow cytometry analyses, HADSCs at P4 showed high expression of the mesenchymal adhesion molecule CD9 and surface marker CD44. CD9 plays an important role in cell adhesion during in vitro culture and acts as a useful marker for evaluating the characteristics and potential of stem cells (8). HADSCs at P4 expressed high levels of CD44, a receptor for hyaluronic acid that aids HADSC migration into the injured tissue during the tissue regeneration process (9). Another specific surface marker for mesenchymal stem cells is CD73, which is not expressed in hematopoietic stem cells, osteoblasts, or osteocytes (10). High expression of CD44 and CD73 in HADSCs has also been reported in previous studies (11–13). A small population of HADSCs at P4 expressed the hematopoietic surface markers CD34, CD45, and CD31. This small percentage of cells is negligible and does not interfere with the multipotent ability of HADSCs (12,14). The culture of HADSCs at P4 was also free of fibroblasts, which are positive for CD34 (15). The low expression of molecular MHC class II (HLA-DP-PQ-DR) suggests the potential of HADSCs for allogenic cell transplantation, due to a lower risk of rejection (16). CD117 is known to play a role in the survival, proliferation and migration of bone marrow stem cells, but it is poorly expressed in HADSCs, as shown by previous work (3). Thus, our results show that after expansion in culture, HADSCs retained stem cell surface marker expression and were unlikely to be contaminated with hematopoietic cells and other differentiated cells (13). Second, after expansion in culture to passage 4, the HADSCs demonstrated the capacity for multilineage differentiation into adipogenic and osteogenic cells. These results further support the data suggesting that the HADSCs expanded in culture in this study retained stem cell characteristics and could be used for cartilage regeneration.
The development of cartilage tissue involves the process of mesenchymal stem cell condensation, aggregation, and differentiation into chondroblasts followed by cell maturation, hypertrophy, calcification, and ultimately cell death (17). Cartilage formation during embryonic development starts with the formation of aggregates of mesenchymal progenitor cells and condensation, which play an important role in creating the microenvironment for chondrogenesis (18). Although the successful chondrogenic differentiation of human mesenchymal stem cells has been reported, the sequential mRNA expression profile has not been examined during the induction stage. In this study, after 24 hours of culture in a chondrogenic induction medium, HADSCs began to attract one another and form cell aggregates. This aggregation denoted the condensation process of the early stage of chondrogenesis. Following cell condensation, HADSCs began to express significantly higher levels of collagen type II, aggrecan core protein, COMP, ELASTIN, and collagen type XI in the first week of induction. In addition, the expression of ELASTIN mRNA in this study showed the feasibility of using differentiated HADSCs for elastic cartilage regeneration, such as in ear (19) and trachea reconstruction (20). The transcription factor SOX9 is an early marker that is expressed during cell condensation to regulate collagen type II and cartilage-specific matrix synthesis (21). Our results showed that continued culture in the chondrogenic induction medium further increased SOX9 expression in HADSCs (compared with the control group), and SOX9 expression reached its highest level at the third week of culture, which could further promote the production of cartilage matrix. Other studies have also shown successful chondrogenic induction upon transfection of SOX9 into human bone-marrow stem cells for cartilage formation (22).
Our study showed that the expression of most of the chondrogenic genes in induced HADSCs (collagen type II, aggrecan core protein, COMP, ELASTIN, and collagen type XI) was reduced at the second and third week of culture. This reduction may have been due to the extracellular matrix mRNA expression returning to the baseline level after the condensation and aggregation process during the first week of induction. Thus, continuous culture of the cells in the chondrogenic induction medium did not promote further extracellular matrix production to increase the size of cell aggregates. These results suggest that chondrogenic induction of stem cells for one week may be sufficient for cartilage regeneration. In addition, chondrogenesis is hypothesized to be regulated by the sequential exposure of the chondrogenic induction factors in a specific timeframe. Therefore, further studies should evaluate the sequential combination of various growth factors to achieve more complete chondrogenesis in HADSCs.
The high expression of a hypertrophy marker, collagen type X, at the third week of chondrogenic induction suggests that the continuous induction of HADSCs could lead the cells toward a hypertrophic state and increased bone matrix synthesis. Several factors have been shown to be related to chondrocyte hypertrophy in vitro, such as the use of the TGF family and dexamethasone (23–24). Therefore, the removal of these two factors in the later period of induction could decrease the hypertrophy process in HADSCs.
Serum-derived growth factors may also limit the continuation of chondrogenesis in stem cells. The ideal chondrogenic induction medium should be totally serum-free such that interference from the serum-derived growth factors can be avoided. However, cells also need proteins such as fibronectin for adherence and other factors from serum to reduce apoptosis (25). Our previous report has shown that supplementation with ITS, IGF-1, bFGF, and TGF-ß2 in the culture medium can promote human chondrocytes to maintain differentiated phenotypes and increased proliferation (26). Furthermore, the formation of good trachea cartilage also requires serum supplementation (20). Human serum from either an autologous or allogenic source is also known to promote articular chondrocyte growth (27). In addition, supplementation with a combination of human serum and bFGF can enhance human nasal septum chondrocyte proliferation and can promote cartilage regeneration (28). Perhaps a serum-free chondrogenic induction medium should also include factors used in chondrocyte culture to reduce cell death and increase chondrogenesis.
HADSCs expanded in culture to passage 4 retained stem cell characteristics and served as a feasible source of cells for cartilage regeneration. Chondrogenesis in HADSCs was most prominent after the first week of chondrogenic induction, but a longer induction period can provoke hypertrophy. Thus, the findings of this study suggest that future studies should investigate the use of HADSCs induced for one week in chondrogenic conditions for cartilage regeneration. This approach could be used in the near future to reduce the time and costs associated with preparing stem cells for the treatment of cartilage lesions.
AUTHOR CONTRIBUTIONSHamid AA was the first author, responsible for performing the experiment, analyzing the data and drafting the manuscript. Idrus RBH and Saim AB were co-supervisors of the project, involved in data analysis and manuscript drafting. Sathappan S was co-researcher in the project, provided specimens and was involved in data analysis. Chua KH was the corresponding author, principal investigator, responsible for the planning of the experiment, supervision of the progress of the experiment, data analysis, and final approval of the manuscript.
This study was made possible by a grant from the Ministry of Science, Technology and Innovation of Malaysia: eScienceFund 02-01-02-SF0290.
No potential conflict of interest was reported.