The aim of this study was to investigate neuropsychological performance and biomarkers of oxidative stress in patients with obstructive sleep apnea and the relationships between these factors.
METHODSThis was an observational, cross-sectional study of 14 patients (36.0±6.5 years old) with obstructive sleep apnea and 13 controls (37.3±6.9 years old). All of the participants were clinically evaluated and underwent full-night polysomnography as well as neuropsychological tests. Blood samples were used to assay superoxide dismutase, catalase, glutathione and homocysteine, as well as vitamins E, C, B11 and B12.
RESULTSThe patients performed poorly relative to the controls on several neuropsychological tests, such as the attention test and tests of long-term memory and working memory/executive function. They also had lower levels of vitamin E (p<0.006), superoxide dismutase (p<0.001) and vitamin B11 (p<0.001), as well as higher concentrations of homocysteine (p<0.02). Serum concentrations of vitamin C, catalase, glutathione and vitamin B12 were unaltered. Vitamin E levels were related to performance in the backward digit span task (F = 15.9; p = 0.002) and this correlation remained after controlling for age and body mass index (F = 6.3, p = 0.01). A relationship between superoxide dismutase concentrations and executive non-perseveration errors in the Wisconsin Card Sorting Test (F = 7.9; p = 0.01) was also observed.
CONCLUSIONSDecreased levels of antioxidants and lower performance on the neuropsychological tasks were observed in patients with obstructive sleep apnea. This study suggests that an imbalance between antioxidants and pro-oxidants may contribute to neuropsychological alterations in this patient population.
Obstructive sleep apnea (OSA) is characterized by recurrent episodes of either total or partial obstruction of the upper airway during sleep, which leads to intermittent hypoxemia, transitory hypercapnia and frequent arousals (1). In addition to cardiovascular morbidity, OSA is associated with a wide spectrum of cognitive symptoms, from mild attention deficits to severe daytime sleepiness (2,3). Several studies have confirmed the presence of neuropsychological alterations in OSA patients (4–6). OSA has a profound impact on psychomotor functioning and cognitive domains, such as attention, memory and executive functioning (7). A decline has been reported in particular cognitive domains, such as attention, long-term episodic memory and working memory/executive function (8–11). Given the complexity of this subject and the potential interactions, a multi-compartment model of working memory has been proposed (12,13). Although intermittent hypoxemia and frequent arousals, either individually or in combination, are involved in these cognitive alterations, the pathophysiology of the neuropsychological deficits has not yet been completely determined (14,15).
The relationship between cognitive dysfunction and oxidative stress has not been extensively investigated, although higher levels of oxidative stress biomarkers have been linked to increased cardiovascular morbidity in OSA individuals (16). However, controversy remains regarding the best marker of oxidative stress. High levels of certain biomarkers, including lipid peroxidation products (17), homocysteine (18), 8–hydroxy-2'-deoxyguanosine in urine (19) and interleukin 10 or TNF-alpha in exhaled air (20), have been reported. Higher-than-normal levels of plasma homocysteine, which is an indirect marker of oxidative stress, have been detected in OSA patients (21). Christou et al. provided further evidence showing that patients with severe OSA have a reduced antioxidant capacity (22). Other studies have indicated that continuous positive airway pressure (CPAP) therapy diminishes plasma oxidative stress (23,24). However, few studies have examined a wider array of antioxidant biomarkers as surrogate measures of oxidative stress in OSA patients.
Preliminary evidence indicates that folic acid and vitamin B12 have anti-apoptotic efficacy and the ability to preserve mitochondrial function (25). Furthermore, vitamin B11 deficiency has been associated with elevated concentrations of homocysteine in the plasma of elderly non-demented patients (26,27). High vitamin B11 (plasma folate) concentrations have been associated with improved global cognitive function (26). However, the association between homocysteinemia and decreased cognition was only observed in participants with low folate levels (28), which suggests potential interplay between these factors. Recently, Singh et al. (2009) determined that both CPAP treatment and antioxidant treatment (oral vitamin E and C) reduced oxidative stress in OSA patients (29). Despite these findings, a relationship between antioxidant biomarkers and cognitive impairment has not been established in OSA patients.
The aim of this study was to investigate neuropsychological performance and oxidative stress biomarkers in OSA patients and to evaluate the relationship between these factors.
MATERIAL AND METHODSStudy Design and SubjectsAll of the subjects were clinically evaluated, underwent neuropsychological tests and polysomnography and had blood samples taken in the morning. The study was approved by the Ethics Committee for Research of the Hospital São Paulo-UNIFESP (# CEP 1266/03), and all of the participants provided written informed consent.
The male patients were consecutively recruited at the Sleep Institute of São Paulo. The inclusion criteria included an age between 25 to 65 years, a body mass index (BMI) <40 and a minimum of 11 years of formal education. All of the cases had confirmed clinical and polysomnographic diagnoses of OSA: apnea-hypopnea index (AHI) above 10 events/h (rather than 5) and at least one symptom or AHI above 15. The exclusion criteria included previous CPAP therapy, the presence of shift work, severe depression, endocrinopathies, (including dyslipidemia, diabetes, obesity or metabolic syndrome), arterial hypertension, anemia, AIDS, current acute myocardial failure or arrhythmia, history of neoplasia, and neurologic or psychiatric disease (including substance/alcohol abuse), as well as the use of hypnotics, neuroleptics, beta-blockers, anti-epileptics, anti-rheumatic medication, steroids and non-steroid anti-inflammatory drugs (NSAIDs), lipid reducers and vitamins. The control group was recruited from the relatives of patients and the employees of the Sleep Institute. The control subjects were matched according to age, sex, weight, scores on the Beck Depression Inventory (30) and years of education, and they were subject to the same inclusion and exclusion criteria as patients, except that they had no sleep disorders (confirmed by polysomnography).
Clinical AssessmentExamination included measurement of weight, height and blood pressure; ectoscopy; neurological examination; ear, nose and throat exam; electrocardiography (ECG) and polysomnography. Sleep complaints were evaluated using a questionnaire that had been adapted for local use, daytime sleepiness was evaluated using the Epworth Sleepiness Scale (ESS) (36) and depressive symptoms were evaluated using the Beck Depression Inventory (30,31). The neuropsychological test battery included measures of various different cognitive functions. Blood samples were collected to assay oxidative stress biomarkers.
PolysomnographyThe recorded parameters included the following: electroencephalogram, electrooculogram, electromyogram (submentonian region and tibialis anterior muscle), electrocardiogram, air flow (recorded by a nasal pressure cannula and a buccal thermistor), respiratory effort by abdominal and thoracic movement (inductance plethysmography), body position, oximetry and snoring (Sonolab Meditron®). The sleep stage scoring (32), respiratory patterns (1), arousals (33) and periodic leg movements were analyzed according to international criteria (34).
Neuropsychological TestsClassical psychometric tests adapted for local use were selected to assess attention, various subcomponents of working memory (including executive functions) and episodic memory. Testing was carried out at approximately 10:00 AM during two sessions that lasted approximately 45 minutes each. The neuropsychological measures were investigated using the Toulouse-Piéron Attention Test (35,36), the Wisconsin Card Sorting Test (WCST) for executive functions (37,38), the Digit Symbol Substitution Test (39) for the processing of visual figures, the forward Digit Span measuring the functioning of phonological storage within working memory and the backward Digit Span evaluating executive functions. Other tests included the Similarities Test to evaluate abstract verbal reasoning, the Logical Memory and Verbal Paired Association Tests to evaluate episodic memory (immediate recall short-term verbal memory) and long-term memory (delayed recall long-term verbal memory) (13), and the immediate and delayed recall of the Rey-Osterrieth Complex Figure Test (40,41) to evaluate short- and long-term visual non-verbal memory, respectively, as well as planning and perceptual organization.
Biomarkers of Oxidative StressFor the biochemical analyses, venous blood was collected in the morning, at approximately 8:00 AM after 12 hours of fasting. The red blood cells were washed and hemolyzed to assay the antioxidant enzymes and glutathione, whereas the plasma was used for the vitamin and amino acid assays. Two enzymatic antioxidants were assayed, i.e., superoxide dismutase (SOD) (42) and catalase (43), in addition to three other non-enzymatic antioxidants, i.e., glutathione (44) and vitamins B11 and B12 (45).
Statistical AnalysisDescriptive statistics are presented as the mean±standard deviation, range and frequency (% values). Fisher exact tests for categorical variables, the Mann-Whitney U test for continuous variables and Student's t test for normally distributed data with equal variances were performed to compare the cases and controls. Linear regression analysis was used to examine the relationship between scores of neuropsychological tests (dependent variables) and the levels of biomarkers for oxidative stress. Posterior adjustments for age and BMI were performed. The level of significance was set at p<0.05. Analyses were conducted using the Statistical Package for Social Sciences V16.0 [SPSS Inc., Chicago, IL, USA].
RESULTSIn total, 32 out of 63 consecutively evaluated OSA patients from an outpatient sleep disorder clinic met the inclusion requirements. Of these 32 patients, 18 were initially recruited; the remaining 14 cases were excluded because the presence of shift work (N = 4), the use of medication (NSAIDs and vitamins, N = 1), the presence of arterial hypertension (N = 1), high scores on the Beck Depression Inventory (N = 5), refusal of neuropsychological testing (N = 3) or refusal to participate without a specific reason (N = 4). Fourteen patients who met the eligibility requirements and 13 controls were studied. Thirteen controls were included. The cases and controls were similar with regard to years of schooling (>11 years), age (p = 0.62) and BMI (p = 0.12). As expected, the OSA patients presented more sleep complaints (UNIFESP sleep questionnaire) than the controls (p<0.001) and more daytime sleepiness (ESS, p<0.001). Excessive daytime sleepiness (ESS>10) was more frequent in the OSA patients than in the controls (78.6% vs 15.4%; p = 0.001). Depressive symptoms (according to the BDI) were similar between the cases and controls (p = 0.08).
By definition, the cases presented higher values of AHI than the controls (p<0.001), as well as lower minimum oxygen saturation levels (SpO2 minimum) (p = 0.003) and an increased micro arousal index (p = 0.006) (Table 1).
Polysomnographic results (mean±SD) in controls and patients with obstructive sleep apnea.
| Controls (N = 13) | Patients (N = 14) | p-value | |
|---|---|---|---|
| Clinical data and questionnaires | |||
| Age (years) | 36.0±6.1 | 37.2±6.9 | 0.61 |
| Body mass index (Kg/m2) | 26.9±2.9 | 28.8±3.3 | 0.13 |
| BDI scores | 0.9±1.7 | 2.4±2.5 | 0.08 |
| ESS scores | 1.4±0.5 | 9.6±6.1 | <0.005∗∗ |
| Polysomnography measures | |||
| Sleep efficiency | 87.3±8.2 | 87.8±8∗ | 0.89 |
| S1 (%TTS) | 3.2±1.6 | 6.6±5.1∗ | |
| S2 (%TTS) | 59.3±6.5 | 63.8±11.3∗ | |
| S3 (%TTS) | 15.1±4.5 | 11.3±7.3∗ | |
| REM (%TTS) | 22.4±3.3 | 18.3±8.6∗ | |
| Arousals/h | 7.7±3.4 | 37.9±36.9 ∗∗ | <0.005∗∗ |
| AHI | 1.9±1.5 | 36.4±28.8 ∗∗ | |
| Minimum SpO2 (%) | 88.8±2.6 | 78.0±11.9 ∗∗ | |
Student's t test ∗p<0.05; ∗∗ p<0.01.
The patients performed worse in the classical attention test (Toulouse-Piéron), with significantly more errors (p<0.02). Working memory was also significantly impaired in the patients with regard to retention of the episodic buffer (Logical Memory A+B immediate recollection, p<0.04; Rey Immediate Recall, p<0.001) and executive measures (backward digit span, p = 0.006; similarities, p<0.005; perseveration errors in the WCST, p<0.04).
The patients also showed worse verbal memory performance according to the Logical Memory Delayed Recollection (A+B, p<0.05) and the verbal paired associates delayed recollection (easy plus difficult, p<0.02). Furthermore, reduced values were observed on the Rey Figure Delayed Recall, which is a long-term non-verbal memory test (p<0.001) (Table 2).
Neuropsychological test results (mean±SEM) in the controls and patients with obstructive sleep apnea.
| Neuropsychological Tests | Controls (N = 13) | Patients (N = 14) | p-value |
|---|---|---|---|
| Toulouse-Piéron Correct Answers | 161.6±40.7 | 132.8±29.2 | 0.05 |
| Toulouse-Piéron Errors | 0.07±0.3 | 0.68±0.7 | 0.01∗ |
| Digit Symbol | 55.53±13.1 | 51.7±12.7 | 0.44 |
| Forward Digit Span | 5.85±1.1 | 5.8±0.7 | 0.97 |
| Backward Digit Span | 5.15±0.9 | 4.07±1.0 | 0.006 |
| Similarities | 24.77±1.6 | 21.0±4.0 | 0.004∗∗ |
| Logical Memory–R I (A+B) | 32.54±4.7 | 27.2±7.1 | 0.03∗ |
| Logic Memory–R T (A+B) | 29.92±4.2 | 25.0±7.1 | 0.04∗ |
| Verbal PA–1st Trial | 5.07±1.7 | 4.5±1.5 | 0.36 |
| Verbal PA–2nd Trial | 6.4±1.5 | 6.1±1.4 | 0.56 |
| Verbal PA–3rd Trial | 7.3±0.8 | 6.7±1 | 0.12 |
| Verbal Recollection P A | 7.3±0.8 | 5.7±1.5 | 0.001∗∗ |
| Rey Figure–Copy | 34.4±1.8 | 33.7±2.6 | 0.39 |
| Rey Figure-R I | 29.5±2.9 | 24.0±4.2 | <0.001∗∗ |
| Rey Figure-R T | 29.7±3.6 | 22.3±5.4 | <0.001∗∗ |
| Perseverative Errors | 2.2±5.1 | 6.07±3.8 | 0.03 |
| Failure in Keeping the Set | 0.41±0.9 | 2.1±3.3 | 0.09 |
PA = Paired-Associated; IR = Immediate Recall; LR = Late Recall. Mann-Whitney Test ∗p<0.05 ∗∗p<0.01.
The patients presented lower levels of vitamin E (p<0.006), SOD (p<0.001) and vitamin B11 (p<0.001), as well as higher levels of homocysteine (p<0.02). Serum concentrations of vitamin C, catalase, glutathione and vitamin B12 were equivalent and within the normal range (Table 3).
Oxidative stress parameters (mean±SD) in the controls and patients with obstructive sleep apnea.
| Oxidative stress parameters | Controls (N = 13) | Patients (N = 14) | p-value |
|---|---|---|---|
| Non-enzymatic antioxidant markers | |||
| Vitamin E μmol/L | 19.1±6.7 | 12.8±3.6 | 0.005∗∗ |
| Vitamin C μmol/L | 46.7±14.5 | 49.2±12.7 | 0.63 |
| Glutathione μmol/g Hb | 7.8±1.7 | 7.3±3.8 | 0.70 |
| Enzymatic antioxidant markers | |||
| SOD U/mgHb | 14.4±2.3 | 10±2.9 | <0.001∗∗ |
| Catalase U/mgHb | 11±40 | 99±26 | 0.90 |
| Homocysteine and related vitamins | |||
| Homocysteine u/M | 10.7±2.9 | 16.7±8.0 | 0.02∗ |
| Vitamin B11 ng/ml | 9.3±2.8 | 4.8±2.3 | <0.001∗∗ |
| 480±111 | 464±184 | 0.79 | |
SOD = superoxide dismutase. Student's t test ∗p≤0.05 ∗∗p<0.01.
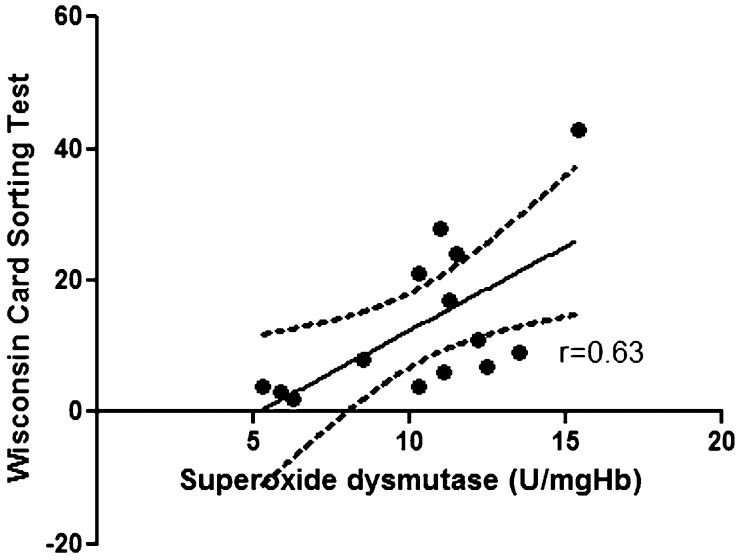
Comparisons between levels of oxidative stress markers and neuropsychological results revealed a correlation between serum concentrations of vitamin E and the results of the backward digit span task (r = 0.76; p = 0.002) in the OSA patients (Figure 1); a similar correlation was not observed in the controls (r = 0.23; p = 0.43). A correlation was observed between the SOD concentration and the executive non-perseveration errors on the WCST in the OSA patients (r = 0.63; p = 0.01) (Figure 2); a similar correlation was not observed in the controls (r = -0.44; p = 0.10). The correlation between vitamin E levels and the backward digit span task remained after controlling for age and BMI (r = 0.69; p = 0.009), as did the correlation between SOD levels and WCST scores (r = 0.63, p = 0.02). No correlations were found among the other oxidative stress parameters, the neuropsychological results and the polysomnographic variables in the OSA patients and in the controls.
The present study, which evaluated a wide range of oxidative stress biomarkers, confirms that oxidative stress is critically elevated in OSA patients. The present study also substantiates an important role for vitamin E and SOD, as it demonstrates a relationship between these antioxidants and neuropsychological performance. It should be noted that the association between OSA and cognitive impairment is complicated by numerous comorbidities, including aging, obesity, genetic factors, hypoxemia, daytime somnolence, cerebrovascular disease and endothelial dysfunction (7). To address this issue, this study considered matched groups of patients and controls, so that the only difference across the groups was a high or low apnea index. It should also be noted that the present study did not find a correlation between neuropsychological results and polysomnographic measures, including oxygen desaturation and/or sleep fragmentation.
To date, various cognitive deficits have been identified in OSA patients. Deficits in attention, vigilance, memory and executive function have all been described. Attempts to explain these alterations have been made using both animal models and patients with OSA. Gozal et al. studied young adult rats and examined the effects of fluctuating ambient oxygenation on learning and neuronal health (46). Based on their results, a model was proposed for a conceptual framework. In this model, sleep fragmentation and/or intermittent hypoxemia and hypercarbia disrupt the restorative features of sleep and consequently disrupt cellular or chemical homeostasis (47). All of these disturbances induce cellular and biochemical stress. Oxidative stress (48), apoptosis-related neural injury, reduced expression of brain-derived neurotrophic factor (BDNF) (49) and molecular alterations (50) have been described in association with neural injury in OSA (51). Consequently, alterations of the cerebral cortex, particularly the pre-frontal cortex, and cognitive dysfunction occur. Neuroimaging studies have confirmed the involvement of the pre-frontal cortex in OSA (52). These alterations have been shown to improve with treatment (53). In addition, recent studies showed that young children with sleep apnea may also undergo neuronal loss and cognitive impairments (54).
Controversy remains regarding the role of hypoxia in cerebral changes related to OSA. For example, most animal models have used fluctuating ambient oxygen patterns to reproduce the chronic intermittent hypoxia associated with OSA. Therefore, the observed brain alterations could merely be secondary to cerebral hypoxia. In contrast to this explanation, sleep fragmentation was recently shown to have an impact on both brain-specific alterations and general metabolism (55). Furthermore, Thomas et al. examined OSA patients using functional magnetic resonance imaging and demonstrated a lower level of activation of the prefrontal cortex while performing a working memory task that was similar in hypoxic and nonhypoxic subjects, which indicates that hypoxia does not influence the cortical dysfunction observed in sleep apnea (56).
Previous studies of the effects of vitamin E on cognition and the role of inflammation and oxidative stress in OSA are controversial. Recently, an uncontrolled study of 20 patients receiving CPAP therapy found that antioxidant intake improved the quality of sleep (29). In addition, CPAP has been reported to improve airway inflammation and oxidative stress (57). Dietary intake of ω-3 PUFA has been associated with lower plasma levels of Aβ42, and this pattern has also been linked with a reduced risk of incident Alzheimer's disease and slower cognitive decline (58). In contradiction to these findings, a metanalysis has shown a lack of evidence for the efficacy of vitamin E in the prevention or treatment of dementia (59). Similar to our findings, increased oxidative stress in the hippocampus and cognitive impairment were previously associated with sleep deprivation and the Western diet (60), which indicates that oxidative stress may present an additional risk factor for the complex cognitive impairment in OSA.
It should be noted that despite the obvious relationship between neuropsychological testing and cognitive function, neuropsychological evaluations describe performance that is not necessarily related to reduced cognition or dementia. In other words, neuropsychological tests should be considered to reflect a cerebral functional state rather than an established brain alteration. As an example of intermittent alterations, studies of seals have recently demonstrated increased levels of endogenous antioxidants to counteract noxious intermittent hypoxemia and chronic cycles of ischemia/reperfusion (61). OSA patients are continuously exposed to intermittent hypoxemia and chronic cycles of ischemia/reperfusion. Thus, it is possible that the adaptive mechanisms observed in seals are species-specific. Other studies may help to corroborate these findings.
This study confirms previous data showing compromised performance in specific neuropsychological tests, particularly in tests measuring attention and memory. Most of previous studies did not examine a wide range of neuropsychological tests. It has recently been noted that relatively little research has specifically examined the influence of OSA on intellectual function (3). Furthermore, in the present study, subjective somnolence was observed in 85% of patients, which agrees with previous studies estimating that 80% of OSA patients present excessive daytime somnolence or some other cognitive alteration (62). Regarding simple attention, a higher number of errors was observed in the Toulouse-Piéron test, which supports a previous report of a significant reduction in attention on various tests (63). Detrimental effects were also observed in episodic and working memory, which corroborates previous findings that these cognitive domains are impaired in OSA patients (64).
With regard to oxidative stress markers, a reduction in the blood concentration of vitamin E was observed, which agrees with the findings in a previous report (65). With regard to enzymatic antioxidants, the OSA patients exhibited a decrease in SOD, which is the first enzyme of the antioxidizing pathway and may represent an oxidant signal. A previous study showed that fMLP-stimulated superoxide release was markedly increased in OSA patients when compared to a control group (66). However, another study found that SOD concentrations were within normal levels in these patients (67). These discrepancies in SOD findings may be the result of methodological differences. Although the current study measured SOD values in erythrocytes, Schulz et al. (2000) analyzed neutrophils (66). Furthermore, other factors (such as age) are known to influence antioxidant measurements. In this study, we observed an increase in homocysteine and a reduction of vitamin B11 but not B12, both of which play fundamental roles in homocysteine metabolism. The vitamin B11 reduction was accompanied by augmented plasma concentrations of homocysteine, as expected (28). Our data are in agreement with another study showing increased homocysteine levels in OSA patients (68). In the present study, blood was obtained from individuals in the morning, at a time when homocysteine concentrations are usually low, and yet concentrations of homocysteine were elevated beyond the normal range.
Some limitations to this study must be acknowledged. This study was an observational cross-sectional evaluation, and a cause/effect relationship among neuropsychological dysfunction, vitamin E and SOD cannot be established. The present evidence is important because it has potential implications for future therapies; however, these data only provide initial evidence, and more experiments to assess the relationship between antioxidants and neuropsychological tests in OSA patients are warranted. In this study, a wide sample of neuropsychological tests and several antioxidant biomarkers was examined. It should be noted that all of the study subjects were male, and the results may not be generalized to women. These results are unique and have not been previously shown. The evidence described here could be corroborated by prospectively studying the effects of administering vitamin E and other antioxidants on neuropsychological test scores in OSA patients.
In conclusion, decreased levels of antioxidants were observed in OSA patients. This study raises the possibility that an imbalance between antioxidants and pro-oxidants may contribute to the neuropsychological alterations observed in OSA patients. To the best of our knowledge, this is the first clinical investigation to relate neuropsychological alterations to oxidative stress biomarkers in OSA.
AUTHOR CONTRIBUTIONSSales LV was responsible for the study design and evaluation, and the collection of clinical data. Bruin VM contributed to the data analysis, manuscript writing and critical review. D'Almeida V was responsible for the biochemical measurements and contributed to the data collection and analysis. Pompeia S contributed to the psychological test design and analysis. Bueno OF contributed to the psychological test design, analysis and critical review. Tufik S contributed to the study design, evaluation and critical review. Bittencourt LR contributed to the study design, analysis, manuscript writing and critical review.
This work was supported in part by the AFIP, FAPESP/CEPID and MCT/CNPq.
No potential conflict of interest was reported.