The purpose of this investigation was to determine whether cognitive awareness of carbohydrate beverage consumption affects exercise-induced lymphocyte apoptosis, independent of actual carbohydrate intake.
INTRODUCTIONCarbohydrate supplementation during aerobic exercise generally protects against the immunosuppressive effects of exercise. It is not currently known whether carbohydrate consumption or simply the knowledge of carbohydrate consumption also has that effect.
METHODSEndurance trained male and female (N = 10) athletes were randomly assigned to one of two groups based on either a correct or incorrect cognitive awareness of carbohydrate intake. In the incorrect group, the subjects were informed that they were receiving the carbohydrate beverage but actually received the placebo beverage. Participants completed a 60-min ride on a cycle ergometer at 80% VO2peak under carbohydrate and placebo supplemented conditions. Venous blood samples were collected at rest and immediately after exercise and were used to determine the plasma glucose concentration, lymphocyte count, and extent of lymphocyte apoptosis. Cognitive awareness, either correct or incorrect, did not have an effect on any of the measured variables.
RESULTSCarbohydrate supplementation during exercise did not have an effect on lymphocyte count or apoptotic index. Independent of drink type, exercise resulted in significant lymphocytosis and lymphocyte apoptosis (apoptotic index at rest = 6.3±3% and apoptotic index following exercise = 11.6±3%, P<0.01).
CONCLUSIONNeither carbohydrate nor placebo supplementation altered the typical lymphocyte apoptotic response following exercise. While carbohydrate supplementation generally has an immune-boosting effect during exercise, it appears that this influence does not extend to the mechanisms that govern exercise-induced lymphocyte cell death.
Acute exercise has a marked effect on the immune system response. Increased neutrophil, monocyte, and lymphocyte concentration have been reported following prolonged exercise,1 moderate intensity walking,2 and short-term swim exercise.3 Two hours of cycling at 55% VO2peak significantly increased neutrophil degranulation and oxidative burst,1 while walking for 30-min at 60% VO2max increased lymphocyte proliferation and plasma interleukin-6 concentration (IL-6).2 Significant increases in tumor necrosis factor-α (TNF-α) have been reported after a 30-min high-intensity run at 85% VO2max4 and following 15-min of moderate-intensity swimming.5 Therefore, it is apparent that aerobic exercise of moderate to high intensity is capable of inducing significant increases in many immune system factors.
While acute exercise significantly increases immune parameters, carbohydrate (CHO) supplementation has been shown to have an immunomodulating effect on many of these responses. CHO supplementation prior to 2.5 h of cycling at 65% VO2max resulted in reduced cell numbers of neutrophils, monocytes, and lymphocytes compared to placebo,6 as well as a significant decrease in plasma IL-6 concentration.7 Similar reductions in immune cell counts and IL-6 have been reported in trained individuals who received CHO supplementation during a 4-h cycle ride at 70% of the individual anaerobic threshold compared to nonsupplemented trials.8
Regarding the lymphocyte response to acute exercise, an increase in cell concentration is observed with the performance of high intensity exercise, followed by a reduction in concentration that is significantly lower than resting levels.9,10 A portion of this post-exercise decrease is likely due to programmed cell death, or apoptosis. Previous research from our laboratory has shown that the apoptotic program in lymphocytes can be induced during exercise,11 and that a significant number of apoptotic cells are in evidence immediately following an exercise bout.12,13 To our knowledge, only one previous study has assessed the effect that CHO supplementation has on lymphocyte apoptosis. Green et al. reported that CHO supplementation at 3.2 grams per kg body weight decreased estimated cell death rates in CD4 and CD8 cells in culture taken from endurance-trained individuals who performed 2.5 hours of cycle exercise at 85% of the anaerobic threshold.14 This finding is consistent with previously cited research that CHO supplementation may reduce the immunoendocrine changes associated with exercise.6-8
A wealth of literature confirms that the central nervous system has a modulatory effect on the immune system during situations of acute stress (see review by Black).15 Neuroendocrine responses to stress are mediated by hypothalamic corticotropin-releasing factor16 which acts on the hypothalamic-pituitary-adrenal axis to increase corticosteroids and catecholamines which tend to exert an immunosuppressive effect.17 In addition, there is evidence of direct innervation of the sympathetic nervous system into primary and secondary lymphoid tissues.18,19 Therefore, it is possible that the immune system can be modified not only by nutritional supplementation as discussed above, but also independently by neural and cognitive factors. The influence of these neural factors may exert an immunosuppressive effect by reducing circulating leukocyte volume, suggesting potential deletion through a cell death mechanism (apoptosis).
While carbohydrate consumption during strenuous aerobic exercise has been reported to reduce the amount of lymphocyte cell death compared to placebo, it is not known whether actual carbohydrate ingestion or simply the cognitive awareness of carbohydrate consumption mediates the aforementioned immune response during exercise. It was hypothesized that knowledge of carbohydrate intake would have no effect on the lymphocyte apoptosis response physiologically induced by aerobic exercise. This would hold true whether participants had a correct or incorrect knowledge of supplementation type. Therefore, the purpose of this investigation was to determine whether knowledge of carbohydrate intake altered the exercise-induced lymphocyte apoptotic response, independent of actual carbohydrate intake.
METHODS AND MATERIALSParticipantsEndurance trained males and females (N = 10, female N = 6, male N = 4) were recruited from local running, cycling, and triathlon clubs voluntarily participated in this investigation. In order to meet inclusion criteria, participants were required to be classified in the “excellent” category for his or her age group for peak oxygen consumption (VO2peak), and were not currently taking any medications.20 Subjects' characteristics are presented in table 1. The Committee for the Protection of Human Subjects at the University of Houston reviewed and approved the testing procedures. Prior to testing, participants reported to the University of Houston's Laboratory of Integrated Physiology, where they were asked to read and sign an informed consent form and complete a medical history questionnaire.
Participant characteristics for correct knowledge (female N = 3, male N = 2) and incorrect knowledge groups (female N = 3, male N = 2).
| Correct | Incorrect | |||||||
|---|---|---|---|---|---|---|---|---|
| Age (yrs) | Height (cm) | Weight (kg) | VO2peak (ml kg-1 min-1) | Age (yrs) | Height (cm) | Weight (kg) | VO2peak (ml kg-1 min-1) | |
| Mean | 33 | 175 | 67 | 58 | 34 | 172 | 72 | 53 |
| SD | 5 | 8 | 8 | 7 | 11 | 9 | 13 | 8 |
Peak oxygen consumption (VO2peak) was determined using an incremental cycle test: stages 1-3 were 3-min in length with 50 watt increases in intensity; subsequent stages were 2-min duration until volitional fatigue. VO2 was determined through automated analysis of expired respiratory gases (Cardio Coach Plus; Salt Lake City, UT). VO2peak was identified as the highest level of VO2 measured and sustained for at least 30-sec. Exercise testing and experimental trials were completed on a Velotron Pro electronically-braked cycle ergometer (RacerMate, Inc.; Seattle, WA) at a self-selected pedal rate.
Subjects were randomly assigned into a group with correct cognitive awareness of supplementation type (female N = 3, male N = 2), or an incorrect knowledge group (female N = 3, male N = 2). For example in the incorrect knowledge group, subjects were informed they were receiving CHO, but actually received the placebo (PLA) beverage; whereas during the during the CHO trial they actually received PLA. The correct cognitive awareness group was informed of receiving CHO on the CHO supplemented trial, as well as placebo during the PLA trial. Each participant completed two experimental trials in a counterbalanced manner: CHO supplementation trial (250 mL every 15-min, 6% CHO beverage) and control trial separated by at least seven days of recovery. Both the CHO and PLA drinks were designed and supplied by the Gatorade Sport Science Institute (Barrington, IL). Drinks were similar in composition, except that the PLA drink lacked carbohydrate. Regardless of supplementation type, participants completed a 60-min ride at ∼80% VO2peak. The environmental conditions (room temperature and 60% humidity) were recorded daily and it was confirmed that there was no significant difference between testing days. All trials were completed between 0600-0800 following an overnight fast and participants were instructed to refrain from exercise for a period of 24-h prior to testing. Participants were counseled not to alter their food intake in the days prior to the experimental trial. Food intake was documented by 24-h food records that were completed by each participant prior to the experimental trial. No significant difference in total calorie and/or macronutrient intake was evident between subjects.
With the exception of the immediate post-exercise sample, subjects were asked to rest for 20-min prior to blood collection and sampling in a quiet, temperature controlled room. Venous blood samples were collected from a peripheral arm vein using a multi-sample butterfly needle into an evacuated tube treated with sodium heparin (BD-Biosciences; City, State). EDTA treated whole blood was used to determine complete blood counts (CBC), and to measure plasma glucose concentration using a YSI 2300 glucose analyzer (Yellow Springs, MO). Venous blood samples were collected before (PRE) and immediately after exercise (POST) and used to make whole blood films within 10-min of collection for the morphological analysis of lymphocyte apoptosis as described previously.12 Briefly, smears were stained in May-Grünwald stain (Sigma-Aldrich, Inc; St. Louis, MO) for 3-5 min, and then washed in phosphate buffered saline (PBS) for 3 min. Afterward, slides were placed in modified Giemsa stain (Sigma-Aldrich, Inc) for 2 min, washed in deionized water, and allowed to air dry before evaluation.
Images of both normal and apoptotic lymphocytes were captured sequentially from the blood films using a digital camera (MA88 Microscope Digital Camera, C&A Scientific Co., Inc.; Manassas, VA) mounted to a light microscope (CXL Plus, Labomed, India) under the oil objective lens. A single investigator evaluated all images in a blinded fashion. According to our previously described procedures, at least 100 cells per slide were captured for subsequent evaluation of apoptotic characteristics.12 Upon evaluation, lymphocytes were considered normal if the cell displayed an approximately circular shape with a smooth cell membrane, while lymphocytes that displayed membrane blebbing or apoptotic bodies were considered apoptotic as has been previously reported in the literature.21 Cell death due to apoptosis was recorded as the apoptotic index (%), and calculated as the number of apoptotic lymphocytes divided by the total number of lymphocytes counted. Slides were counted in duplicate and averaged to give the apoptotic index (AI). If duplicate counts varied by greater than 5%, the third slide was evaluated. The intraclass correlation for a single trained observer using the morphological method to determine exercise-induced lymphocyte apoptosis in our laboratory is R = 0.96.
2.3 Statistical AnalysisBased on previous studies from our laboratory,11-13 a large effect size was anticipated. In an effort to be conservative, our sample size was calculated using a medium effect size with a 25% increase in apoptosis following exercise. The a priori sample size calculation revealed a minimum of 5 subjects to detect an increase in apoptosis with exercise. Post hoc power analysis indicated a statistical power of 92% for the present sample. For power analysis computations, a t-test for the difference between two dependent means was calculated using G*Power software. Statistical analyses were carried out using SPSS 10.1 (SPSS Inc., Chicago, IL). Data were analyzed using a factorial 2 (cognitive awareness: correct or incorrect) × 2 (supplement: CHO or PLA) × 2 (test: PRE or POST) ANOVA with repeated measures on the second and third variable and significance accepted at P≤0.05. Data were tested for normality using the Shapiro-Wilk test.
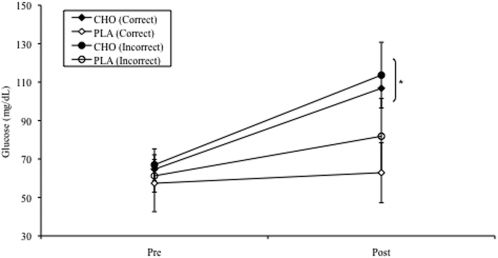
RESULTSGlucoseThere was a significant supplement x test interaction for blood glucose concentration (P = 0.04, Fig 1). Glucose values following exercise in the CHO supplemented conditions were 52% greater than following the PLA trials. Correct or incorrect cognitive awareness of supplement type did not affect glucose concentration.
Glucose measurements (mg/dL) from trained individuals who completed two 60-min cycle ergometry trials at ∼80% VO2peak. Data are presented as group means and standard deviations (bars). One trial was carbohydrate supplemented (CHO), and the other was a placebo trial (PLA). Half of the participants had correct cognitive awareness of the supplementation type (correct) while the other half believed they were receiving the opposite supplementation (incorrect). * indicates a significant difference following exercise (Post) in the CHO supplemented trials (P = 0.04).
No interactions were evident with regard to lymphocyte counts. The only significant main effect was for test, where after exercise there were 93% more lymphocytes (P<0.001, Fig 2).
Lymphocyte concentration before (Pre) and immediately after (Post) 60-min of cycle ergometry at ∼80% VO2peak in carbohydrate (CHO) and placebo (PLA) trials. Data are presented as means and standard deviations (bars). One trial was carbohydrate supplemented (CHO), and the other was a placebo trial (PLA). Half of the participants had correct cognitive awareness of the supplementation type (correct) while the other half believed they were receiving the opposite supplementation (incorrect). * indicates significant increase with exercise compared to the resting condition (P<0.001).
Cognitive awareness of drink type did not affect the lymphocyte cell death response before or after exercise (P = 0.43). There was a significant main effect of exercise on lymphocyte apoptosis, where after exercise there was an 84% increase in lymphocyte apoptotic index (P<0.01, Fig 3).
Apoptotic index (%) in lymphocytes before (Pre) and after (Post) 60-min of cycle ergometry at ∼80% VO2peak in carbohydrate (CHO) and placebo (PLA) trials. Data are presented as means and standard deviations (bars). One trial was carbohydrate supplemented (CHO), and the other was a placebo trial (PLA). Half of the participants had correct cognitive awareness of the supplementation type (correct) while the other half believed they were receiving the opposite supplementation (incorrect). * indicates significant increase with exercise compared to the resting condition (P<0.01).
The main finding of this investigation was that cognitive awareness of drink type had no physiological effect on selected immune parameters following high-intensity aerobic exercise. Individuals who had correct cognitive awareness of consumed supplementation (CHO or PLA) had statistically similar measures for plasma glucose, lymphocyte concentration, and lymphocyte apoptosis compared to individuals who had an incorrect cognitive awareness of the ingested supplement. In addition, we observed no effect of CHO supplementation on either lymphocyte concentration or apoptotic index following 60-min of cycle exercise at 80% VO2peak.
Carbohydrate supplementation significantly increased plasma glucose following exercise regardless of correct or incorrect cognitive awareness of drink type. The rise in glucose response has been confirmed by a number of other investigations.14,22–24 We are aware of only one other deception study in which subjects have received an incorrect cognitive awareness of carbohydrate or placebo supplementation prior to exercise.25 As we have shown, cognitive awareness of drink type (whether correct or incorrect) did not have an effect on the blood glucose response. The lack of a difference likely means that stimulating factors facilitating the release of glucose into the bloodstream (i.e. glucagon, epinephrine, etc.) are not affected directly by cognitive awareness during a bout high-intensity aerobic exercise.
An exercise-induced lymphocytosis was evident following both CHO and PLA supplemented bouts. An increase in lymphocyte concentration following exercise has been reported in numerous investigations.1–3,5–6 Thirty minutes of walking at 60% VO2max increased lymphocytosis by 28% in human subjects2 and 120-min of cycling at 65% VO2max resulted in a 20% increase.6 Using an animal model, Prestes et al. observed increases in lymphocyte counts of 98% and 165% in male Wistar rats who performed unloaded swimming for 5 and 15-min, and increases of 252% and 191% in animals who swam for 5 and 15-min with a 5% load.5 The magnitude of lymphocytosis in the present study was 93% in endurance trained participants who completed 60-min of cycle ergometry exercise at 80% VO2peak. An explanation for the lymphocytosis observed with exercise of increasing intensity is the greater expression of adhesion/activation molecules which can serve to facilitate movement of lymphocytes from the marginal pools into the circulation.26,27
While others have reported a significant decrease in lymphocyte count with CHO supplementation compared to PLA,6 our results did not show a difference. As part of an investigation to assess the effect of carbohydrate supplementation following caffeine ingestion, the participants of Walker et al. consumed either caffeine or placebo prior to exercise, and either carbohydrate or placebo during a 2 h cycle ride at 65% VO2max.6 Using only the trials in which placebo was consumed prior to exercise (and excluding the effect of caffeine), it was reported that supplementation with CHO during exercise reduced lymphocyte count by 21% compared with the trial in which placebo was administered during exercise.6 The common explanation for immunomodulation associated with CHO supplementation are effects through hypothalamic-pituitary-adrenal (HPA) activation and a muted stress hormone response.28 In the present study, CHO was only ingested prior to exercise and did not result in suppressed lymphocyte cell count compared to PLA. There are two possibilities to explain the differences between the study by Walker et al.6 and our findings. First, our participants completed only a 1h cycle ride compared to 2 h. It is possible that the extended exercise duration differentially affected the immune response through increased HPA activation compared to our subjects who completed only half of the duration. Second, the combination of supplemented glucose during the 2 h cycle exercise by the subjects of Walker et al. could have resulted in a greater prolonged effect toward reducing immune parameters compared to our participants who received a single bolus of carbohydrate prior to exercise, but did not receive supplementation at any other point during their exercise trial. Thus it is possible that blood glucose concentration plays a modulating role on cells of the immune system during exercise. In addition, it is likely that CHO has a different effect depending on the immune cell subset. While an overall decrease in leukocyte numbers have been reported in various CHO supplementation investigations,8,24-25 ingestion appears to affect neutrophils to a greater extent than lymphocytes. Nieman et al. and Sharhag et al. both found carbohydrate supplementation to reduce neutrophil count, but to have no effect on numbers of lymphocytes.8,24
Neither correct, nor incorrect, cognitive awareness of drink type altered immunity in terms of lymphocyte apoptosis following exercise. To our knowledge, only one other study has attempted to evaluate the effect of carbohydrate supplementation on exercise-induced lymphocyte cell death.14 Green et al. used carboxyfluorescein succinimidylester fluorescence to estimate lymphocytes undergoing mitosis or cell death using iterative processes until all cells could be accounted for.14 They reported the estimated cell death rates of T-cell subsets to be significantly reduced in CHO supplemented trials compared to PLA bouts in six well-trained male cyclists. In the present study, carbohydrate supplementation did not have an effect on lymphocyte apoptosis. Recently, exercise-induced lymphocyte apoptosis has been measured using either biochemical markers,4,29–30 or a morphological technique.11-13 In the current investigation, we employed the aforementioned morphological method which we have proposed is potentially more sensitive to cells displaying a wider range of apoptotic characteristics compared to a single apoptotic biomarker. We acknowledge that this method does have drawbacks (i.e. highly subjective, low number of cells that are evaluated per sample, etc.); however the methodology was the gold standard to which the current biomarker techniques were validated against.31 Using a direct evaluation of cells displaying morphological characteristics of apoptosis, we found that carbohydrate supplementation during exercise did not alter exercise-induced cell death compared to the placebo supplemented trial. While carbohydrate supplementation may alter certain immune parameters, it appears that ingestion has no effect on the mechanisms governing cell death induction during exercise.
While we were unable to ascertain differences in select immune parameters due to cognitive awareness of carbohydrate in the present investigation, our study design could be modified to make detection of such differences more likely. The utilization of a single group cross-over intervention in which subjects randomly complete the conditions of exercise alone, exercise with carbohydrate supplementation, and exercise in conjunction with placebo, would represent an acceptable experimental design in this regard. It should also be noted that the findings of the present study can only be applied to young and middle-aged individuals who are highly aerobically trained, and should not be extended to other populations. In this regard, other investigations are warranted.
In conclusion, our findings indicate that cognitive awareness (either correct or incorrect, of carbohydrate or placebo) has no effect on blood glucose concentration, lymphocyte concentration, or exercise-induced lymphocyte apoptosis. While glucose intake itself may reduce the magnitude of various immune responses, knowledge of supplementation and indeed the carbohydrate supplement itself has no effect on altering immunity in terms of lymphocyte cell death following exercise.
AcknowledgementsThe authors would like to recognize Western Kentucky University's Faculty Scholarship Council for support through a Summer Faculty Award. In addition, the authors would like to thank Michael Kueht and Nisa Dadjoo for their technical assistance with the investigation.