Recent studies have shown that circulating microRNAs might be useful, novel biomarkers for the diagnosis of acute myocardial infarction. The aims of this study were to evaluate the expression of cardiac-specific miRNAs (miR-1, -133a, -208b, and -499) in patients with acute myocardial infarction and to compare the diagnostic values of these miRNAs with that of cardiac troponin T.
METHODS:Sixty-seven plasma samples obtained from patients with acute myocardial infarction and 32 plasma specimens collected from healthy volunteers were analyzed in this study. The levels of cardiac-specific miRNAs (miR-1, -133a, -208b, and -499) were measured by quantitative reverse transcription-polymerase chain reaction, and the concentrations of plasma cardiac troponin T were measured using electrochemiluminescence-based methods and an Elecsys 2010 Immunoassay Analyzer.
RESULTS:The levels of plasma miR-1, -133a, -208b, and -499 were significantly higher in acute myocardial infarction patients (all p<0.001) than in healthy volunteers. The expression of the cardiac-specific miRNAs in acute myocardial infarction patients decreased to close to the baseline levels at the time of hospital discharge (all p>0.05). There were no correlations between the levels of the four circulating miRNAs and the clinical characteristics of the study population (all p>0.05). Furthermore, receiver operating characteristic curve analyses showed that the four plasma miRNAs were not superior to cardiac troponin T for the diagnosis of acute myocardial infarction (all p>0.05).
CONCLUSION:Our results demonstrate that circulating miR-1, -133a, -208b, and -499 may be useful biomarkers in acute myocardial infarction patients but that these miRNAs are not superior to cardiac troponin T for the diagnosis of acute myocardial infarction.
Acute myocardial infarction (AMI) is a leading cause of morbidity and mortality worldwide, and approximately three to four million people are estimated to suffer from AMI each year (1). Early reperfusion can reduce the mortality rate of AMI (2); therefore, the timely diagnosis of AMI is critical.
The circulating levels of cardiac troponins (cTns) are considered the “gold standard” for the early diagnosis of AMI because cTns are both highly specific and sensitive for cardiac injury (3). However, early studies have reported that cTn concentrations are also elevated in patients with end-stage renal disease (ESRD) and that these molecules might serve as biomarkers for renal failure (4,5). Therefore, it is essential to explore new biomarkers with greater specificity for AMI.
MicroRNAs (miRNAs) are a class of endogenous, non-coding single-stranded small RNAs of 19 to 25 nucleotides in length (6) that can regulate gene expression and play critical roles in various pathophysiological processes (7,8). miRNAs generally suppress gene expression by inhibiting mRNA translation or inducing mRNA degradation. Each miRNA can regulate several distinct target mRNAs, and conversely, single mRNAs can be targeted by several distinct miRNAs (9). Thus, the overall effects of miRNA-associated regulation can be very complex. To date, over 700 human miRNAs have been cloned or sequenced (10,11). Although the biological and pathological functions of miRNAs are not completely understood, it is clear that many miRNAs demonstrate tissue- or cell-specific distributions and play important roles in cell and/or tissue functions under various conditions (12,13). Furthermore, recent studies have demonstrated that miRNAs can be detected in the peripheral blood or plasma, and that the levels of circulating miRNAs are characteristically altered in individuals with various pathological conditions, indicating that circulating miRNAs may be useful diagnostic biomarkers for specific diseases (14,15).
Interestingly, miRNAs are present in the blood and/or plasma in a strikingly stable form that withstands even repeated freeze/thaw cycles and is resistant to RNase degradation (16). Although the mechanisms by which miRNAs are released into the circulation are not clear, the levels of several miRNAs in the blood and/or plasma were observed to be altered during AMI. This result suggests that circulating myocardial-derived miRNAs, such as miR-1, 133a, -208b, and -499, might be good biomarkers for the diagnosis of AMI (17-25). However, the suitability of these four circulating miRNAs for the diagnosis of AMI in humans remains somewhat controversial. For example, Wang et al. found that circulating miR-208b was a good biomarker for the diagnosis of AMI, whereas circulating miR-1, -133a, and -499 were not good biomarkers because of their low sensitivities (17). Meanwhile, Alessandra et al. have reported that circulating miR-208b could not be detected in AMI patients and that circulating miR-1, -133a, and -499 are novel biomarkers for the diagnosis of AMI (25).
Therefore, the objectives of the current work were to measure the levels of circulating miRNAs (miR-1, -133a, -208b, and -499) and the concentration of cardiac troponin T (cTnT) in AMI patients at two medical centers. Additionally, we analyzed the utility of these four miRNAs as novel biomarkers for the early diagnosis of AMI and compared their diagnostic values with that of cTnT.
MATERIALS AND METHODSClinical specimensSixty-seven patients diagnosed with AMI were enrolled in this study at the First Affiliated Hospital of Sun Yat-Sen University (Guangzhou, China) and the First Affiliated Hospital of NanHua University (Hengyang, China) between October 2010 and March 2011. The inclusion criteria for patients with AMI were based on the redefined guidelines from the most recent standard definition of MI (26). Briefly, AMI was diagnosed in patients with increased cTnT or creatine kinase-MB (CK-MB) levels combined with chest pain lasting >30 minutes and electrocardiogram (ECG) findings such as new pathological Q waves or ST-segment elevation or depression. Patients were excluded if they had received intravenous thrombolytic or anticoagulant treatment before the initial blood samples were acquired. Forty-four patients (66%) experienced ST-segment elevation myocardial infarction (STEMI), whereas 23 (34%) experienced non-ST-segment elevation myocardial infarction (NSTEMI). Thirty-nine patients (58%) were treated with percutaneous coronary intervention (PCI), whereas 28 (42%) were treated with thrombolysis. Thirty-two age- and sex-matched samples from healthy volunteers (no history of cardiovascular disease and normal electrocardiographic findings) were used as controls. The study protocol was approved by the Ethics Committees of Sun Yat-Sen University and NanHua University, and written informed consent was obtained from all participants.
Plasma collection and storageVenous blood samples (3 mL) were collected from AMI patients on Day 1 (n = 67) within 12 h of the onset of symptoms (4.6±2.9 h) and on Day 14 (n = 18) and from healthy volunteers using K2-EDTA-coated tubes. Plasma was isolated by centrifugation at 1,000 g for 10 minutes at 4 °C, followed by centrifugation of the supernatant at 14,000 g for 15 minutes at 4 °C. The plasma was transferred into RNase/DNase-free tubes and stored at -80 °C until RNA extraction.
RNA isolationTotal RNA was isolated from plasma using the TRIzol LS® reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions and dissolved in 10 μL of diethylpyrocarbonate (DEPC)-treated water. The concentration and quality of the RNA samples were determined using a BioPhotometer (Eppendorf).
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)The Bulge-Loop™ miRNA qRT-PCR Primer Set (RiboBio Co, Guangzhou, China) was used to detect and quantify the expression of mature microRNAs (miR-1, -133a, -208b, and -499) according to the manufacturer’s instructions; a Caenorhabditis elegans microRNA (cel-miR-39) was used as the internal control (14). Total RNA (0.5 μg) from each sample was reverse transcribed using microRNA-specific primers and M-MLV reverse transcriptase (Promega, Madison, WI, USA). The reaction was carried out for 60 min at 42 °C, followed by 10 min at 70 °C, and the resulting cDNA was stored at -20 °C until use.
The qRT-PCR reactions were performed on the PRISM 7900HT sequence detection system (Applied Biosystems, Carlsbad, CA, USA) in 20-μL reactions containing 2 μL of reverse transcription product, 2 μL of PCR forward primer (5 μM), 2 μL of Universal Adaptor PCR Primer (5 μM), 9 μL of Platinum SYBR Green qPCR SuperMix-UDG reagent (Invitrogen, Carlsbad, CA, USA), and 5 μL of ddH2O. The reactions were incubated at 95 °C for 2 min, followed by 40 cycles of 95 °C for 15 s, 60 °C for 30 s and 95 °C for 15 s. The samples were then heated from 60 °C to 95 °C to obtain melting curves. Reactions containing either no reverse transcriptase or no template were used as negative controls, and all assays were performed in triplicate. These procedures were performed by an investigator (L.L.) blinded to the clinical characteristics of the patients. The threshold cycle (Ct) was defined as the fractional cycle number at which the fluorescence exceeded the given threshold, and the relative expression values of the microRNAs were calculated using the 2−ΔΔCt method (27).
cTnT determinationThe levels of plasma cTnT were determined using electrochemiluminescence-based methods with the Elecsys 2010 Immunoassay Analyzer (Roche Diagnostics, Switzerland) according to the manufacturer’s protocol.
Statistical analysisThe Statistical Package for Social Sciences, version 16.0 (SPSS, Inc., Chicago, IL, USA), was used for the statistical analysis. All data are presented as the mean±standard deviation unless otherwise noted. The significance of the differences in clinical characteristics between AMI patients and controls was tested using Student’s t-test or Fisher’s exact test. The Mann-Whitney test was conducted to compare the expression of microRNAs between the AMI patients and controls. The microRNA levels measured in each AMI patient at the two time points were compared using the Wilcoxon signed rank test. Receiver operating characteristic (ROC) curves were established to discriminate between AMI patients and controls with Stata version 10.0 and were compared using the chi-square test. All p-values were two-tailed, and p<0.05 was considered statistically significant.
RESULTSClinical characteristics of the study populationIn the present study, we enrolled 67 patients with AMI and 32 controls. The clinical characteristics of each group are listed in Table 1. The mean age of the AMI patients was 63.84±11.17 years, and the mean age of the controls was 61.75±9.58 years (p = 0.332). Both groups were predominantly male (52/67 in the AMI group and 22/32 in the control group; p = 0.343). The mean cTnT level at admission in AMI patients was 1.30±1.06 ng/mL, which was significantly higher than the mean cTnT level in the control group (p<0.001). There were no significant differences in any other clinical characteristics between the AMI and control groups.
Clinical characteristics of the study population.
| Characteristics | Control (n = 32) | AMI (n = 67) | p-value |
|---|---|---|---|
| Age (years) | 61.75±9.58 | 63.84±11.17 | 0.332 |
| Male/Female (n/n) | 22/10 | 52/15 | 0.343 |
| Currently smoking, n (%) | 13 (40.63) | 32 (47.76) | 0.505 |
| Diabetes mellitus, n (%) | 5 (15.63) | 13 (19.40) | 0.649 |
| Hypertension, n (%) | 15 (46.88) | 38 (56.72) | 0.358 |
| Hyperlipidemia, n (%) | 14 (43.75) | 36 (53.75) | 0.353 |
| Systolic blood pressure (mmHg) | 125.56±12.52 | 129.66±15.35 | 0.203 |
| Diastolic blood pressure (mmHg) | 79.75±6.81 | 81.57±9.76 | 0.210 |
| Cardiac troponin T (ng/mL) | 0.03±0.02 | 1.30±1.06 | <0.001 |
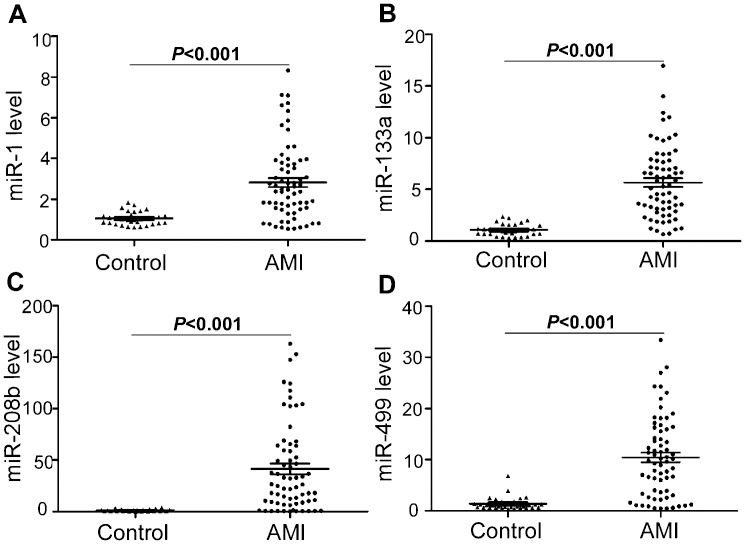
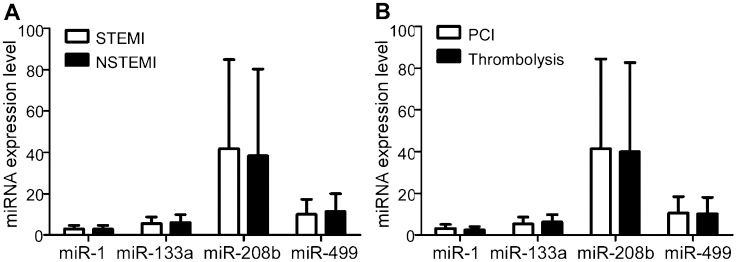
To determine whether the circulating cardiac-associated microRNAs were associated with the onset of AMI, we investigated the plasma levels of four microRNAs (miR-1, -133a, -208b, and -499) in AMI patients and controls. As shown in Figure 1 the plasma concentrations of miR-1, -133a, -208b, and -499 were all markedly elevated in AMI patients relative to controls (p<0.001). Furthermore, there were no significant differences in the levels of these four miRNAs between STEMI and NSTEMI patients (all p>0.05, Figure 2A, and there were also no significant differences between the PCI and thrombolysis groups (all p>0.05, Figure 2B.
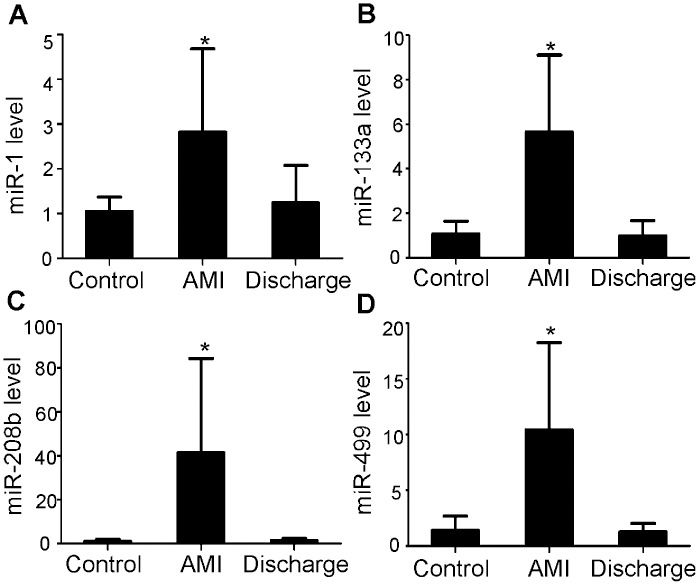
We also measured the levels of the four miRNAs in plasma from AMI patients at the time of hospital discharge (n = 18, patients who lived more than 14 days and agreed to take part in this part of the study), and we found that the miRNA levels had decreased to baseline levels, i.e., they were not significantly different from the levels in the controls (p>0.05) (Figure 3).
Alteration of the plasma miRNA levels in AMI patients. (A-D) The levels of miR-1, -133a, -208b, and -499 were markedly increased in plasma samples gathered within 12 hours of the onset of AMI (Day 1). At the time of hospital discharge (Day 14), the levels of these four miRNAs had returned to levels similar to those in the control subjects (n = 32 control subjects, n = 67 AMI patients at Day 1, n = 18 AMI patients at Day 14). The values shown represent the fold change of each miRNA in AMI patients relative to control subjects. The results are presented as the mean ± standard deviation. ∗p<0.001 vs. control.
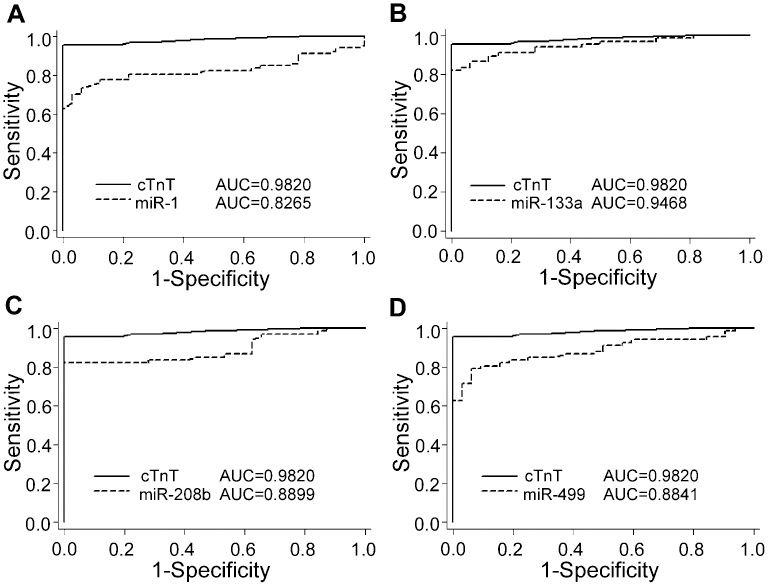
To evaluate the predictive power of circulating cardiac-associated microRNAs for AMI, we performed ROC analysis for 67 patients with AMI. As shown in Figure 4 the areas under the ROC curves (AUCs) for miR-1, miR-133a, miR-208b, and miR-499 were 0.8265 (95% confidence interval, 0.7441-0.9088), 0.9468 (95% confidence interval, 0.9057-0.9879), 0.8899 (95% confidence interval, 0.8259-0.9540), and 0.8841 (95% confidence interval, 0.8187-0.9495), respectively. The AUC measured for cTnT was 0.9820 (95% confidence interval, 0.9289-0.9975). These results demonstrate that the four miRNAs had marked sensitivity and specificity for AMI. However, none of the four miRNAs tested was superior to cTnT for the diagnosis of AMI (all p>0.05).
Comparisons of the sensitivity and specificity of the four plasma miRNAs and cTnT for the diagnosis of AMI. Receiver operating characteristic (ROC) curves were constructed to evaluate the diagnostic values of miR-1, -133a, -208b, and -499 for AMI patients in comparison with cTnT. AUC, area under the ROC curve.
In our present study, we found that the levels of miRNA-1, -133a, -208b, and -499 were significantly increased in patients after AMI relative to the levels in healthy volunteers, and our data indicated that the elevated expression of these four miRNAs was coincident with the progression of the AMI. As we now know, in the early stages of AMI, pathological changes such as myocardial ischemia, hypoxia, edema, and necrosis occur rapidly, followed by the release of necrotic products, such as cardiac troponins (cTns), creatine kinase (CK) and brain natriuretic peptide (BNP), into the bloodstream. These established biomarkers can be useful for the early diagnosis of AMI. The results of the present work suggest that miRNA-1, -133a, -208b, and -499 might leak out of the necrotic myocardium and into the circulation during the early stages of AMI, and the levels of these miRNAs would thus become elevated as the AMI progresses; therefore, these four circulating miRNAs might be useful biomarkers for the diagnosis of AMI.
AMI is a leading cause of death in both developed and developing countries, and the mortality rate of this disease can be reduced by early diagnosis. Established biomarkers such as cTnT, CK-MB, BNP and C-reactive protein (CRP) play critical roles in the diagnosis of AMI (3,28,29).
According to the recent redefinition of myocardial infarction by the ESC/ACC (European Society of Cardiology/American College of Cardiology) Consensus group, troponins are more sensitive and specific measures of myocyte necrosis with respect to AMI diagnosis than CK-MB, BNP, and CRP (30-32). Nevertheless, cTns have several weaknesses, including incomplete specificity for AMI. For example, some studies have reported that cTn levels were elevated in ESRD patients and that cTns might serve as prognostic biomarkers in RF patients (4,5). Therefore, it is important to continue the search for new biomarkers with higher sensitivity and specificity for the early diagnosis of AMI.
Several recent studies have reported that miRNAs exhibit tissue- and cell-specific characteristics under various conditions; additionally, miRNAs can be released into the circulation, where they persist in a stable form (12,16,17). Several consecutive reports have shown that the levels of circulating, cardiac-specific miRNAs were altered in conjunction with the progression of cardiovascular diseases; therefore, these miRNAs might be promising novel biomarkers for the diagnosis of AMI.
The findings from our present study are consistent with those reported in recent articles, indicating that levels of the circulating miRNAs miRNA-1, -133a, -208b, and -499 were increased during AMI and were significantly higher than those in healthy volunteers. The levels of the four circulating miRNAs decreased to normal levels at the time of hospital discharge. We also analyzed the concentration of cTnT during AMI. cTns are established biomarkers for the diagnosis of AMI and reflect infarct size. Compared with cTnI, cTnT is more specific and sensitive for the diagnosis of AMI (33-35). Therefore, we selected cTnT as a benchmark for comparison with circulating miRNAs in the present study.
We performed ROC curve analyses to determine the diagnostic values of the four circulating miRNAs and to compare them with cTnT. The results of these analyses indicated that circulating miR-1, -133a, -208b, and -499 were specific and sensitive for the early diagnosis of AMI and are promising novel biomarkers for AMI. Although there were positive relationships between the four circulating miRNAs and cTnT within 12 hours of the onset of symptoms (Day 1), none of the four circulating miRNAs (miR-1, -133a, -208b, and -499) was superior to cTnT for the early diagnosis of AMI.
According to these results, cTnT seems to be more sensitive and specific than any of the four circulating miRNAs in patients with AMI. We hypothesized that cTnT might be released from necrotic myocardium at the time of the AMI in patients prior to the onset of chest pain. Previous studies have demonstrated that cTnT is primarily bound to myofibrils and that miRNAs are primarily bound to cytosolic protein complexes. We hypothesized that these differences might affect the patterns of cTnT and miRNA release during the progression of myocardial necrosis.
Our present study provides clinical evidence to support the use of circulating miR-1, -133a, -208b, and -499 as biomarkers for AMI. However, our work has some limitations, including the small sample size. Therefore, additional studies with larger cohorts of healthy volunteers and patients are needed to definitively demonstrate the diagnostic value of the four circulating miRNAs as practical biomarkers in comparison with other markers and to reduce the rate of false-positive results. Additionally, the diagnostic values of the circulating miRNAs need to be investigated in further studies involving ESRD patients with AMI and in AMI patients without ESRD.
In conclusion, this study verified the diagnostic value of four circulating miRNAs (miR-1, -133a, -208b, and -499) in the early stage of AMI and compared them with the established biomarker cTnT. We demonstrated that miR-1, -133a, -208b, and -499 might serve as novel biomarkers for the diagnosis of AMI; however, the diagnostic values of these circulating miRNAs were not superior to that of cTnT. Because the cTnT concentration is also increased in ESRD patients, we propose that cTnT and miRNA analyses could be combined to enhance the specificity of AMI diagnosis.
This study was supported by grants from the National Natural Science Foundation of China (81071030), the Science and Technology Foundation of Guangdong Province, China (2010B031600089, 2011B080701006), and the Training Foundation for the Youth Scholars of Sun Yat-sen University (09ykpy31). The funders played no role in the study design, data collection, data analysis, decision to publish or preparation of the manuscript.
No potential conflict of interest was reported.
Li YQ, Zhang MF and Wen HY contributed equally to the study. Li YQ, Li X, and Zhang MF helped performing the research and data analysis and writing the manuscript. Hu CL, Wei HY, Ai CM, and Wang G contributed to the recruitment of patients and volunteers. Liu R helped performing the experiments. Corresponding authors (Li X and Liao XX) conceived and designed the study. All authors have approved the final manuscript.