Renal dysfunction is common during episodes of acute decompensated heart failure, and historical data indicate that the mean creatinine level at admission has risen in recent decades. Different mechanisms underlying this change over time have been proposed, such as demographic changes, hemodynamic and neurohumoral derangements and medical interventions. In this setting, various strategies have been proposed for the prevention of renal dysfunction with heterogeneous results. In the present article, we review and discuss the main aspects of renal dysfunction prevention according to the different stages of heart failure.
Renal dysfunction is common during episodes of acute decompensated heart failure and is found in up to 64% (1) of patients at hospital admission. Historical analysis indicates that the mean creatinine level at admission has risen in recent decades (2). Different mechanisms underlying this change over time have been proposed. First, as a consequence of better clinical care and surgical interventions, there has been a shift towards a larger number of patients with advanced heart failure (3), and in these circumstances, patients tend to be older and to accumulate risk factors, such as hypertension and diabetes mellitus (4,5). Additionally, hemodynamic and neurohumoral derangements are exacerbated during episodes of decompensation and further contribute to de novo kidney dysfunction or the worsening of a chronic kidney disease. Medical interventions may also produce a reduction of the glomerular filtration rate (GFR) by reducing blood pressure, inducing hypovolemia or reducing the glomerular perfusion pressure.
However, in biological phenomena, epidemiological associations may not reflect causality, and whether worsening renal function itself contributes to the increased mortality or whether it merely serves as a marker of a more severe disease remains unclear. Conditions such as diabetes and hypertension may offer an epidemiological link that associates heart failure and kidney disease. Additionally, kidney disease and heart failure have been suggested not to represent single clinical entities but rather to represent manifestations of a broader vascular injury associated with aging that affects multiple organs (6).
In this setting, various strategies have been proposed for the prevention of renal dysfunction with heterogeneous results. In the present article, we review and discuss the main aspects of renal dysfunction prevention according to the different stages of heart failure.
DefinitionsIn 2005, the American Heart Association and the American College of Cardiology proposed a staging classification that incorporated a conceptual change relative to the classical definition of heart failure syndrome, which dealt almost exclusively with symptomatic patients. The new categorization incorporated stages A and B, with stage A identifying patients at risk for heart failure but without structural heart disease or symptoms of the syndrome and stage B encompassing patients with structural heart disease but without symptoms of heart failure. During the course of the syndrome, patients may experience a decrease in kidney function, and strategies for treatment and prevention should take into consideration the burden of epidemiological characteristics and the presence of risk factors in the context of the hemodynamic and neurohumoral events associated with heart failure syndrome.
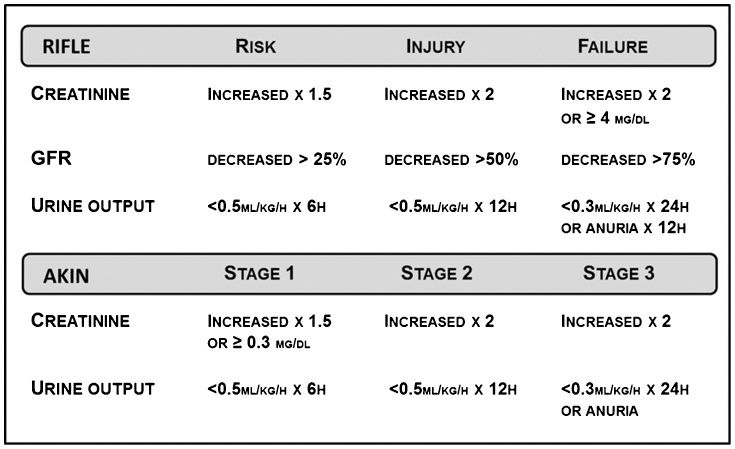
Patients with heart failure who are experiencing a decrease in renal function usually have peculiar characteristics, including manifestations of hypervolemia, oliguria and diuretic resistance, in a clinical picture frequently referred to as cardiorenal syndrome. Cardiorenal syndrome has been suggested to encompass disorders of the heart and kidneys, either acute or chronic, in such an association that dysfunction in one organ may induce acute or chronic dysfunction of the other. A categorization into five different types has been proposed (7). Although such a categorization may be helpful in identifying patients with different pathophysiological mechanisms, it includes a heterogeneous group of clinical presentations or diagnoses under the same definition and adds little value to the care of patients with a condition in which the mechanisms remain largely unknown and for which a precise diagnosis may still be elusive in clinical practice. The AKIN and RIFLE criteria have been proposed to stratify patients at risk for the occurrence of renal dysfunction (Figure 1. However, it should be acknowledged that these criteria are mostly derived from cohorts of patients in heterogeneous clinical conditions and represent a general approach to critically ill patients that including cardiac surgery patients, burn patients and liver and bone marrow transplantation patients.
Furthermore, a subset of patients who experience an increase of 0.3-0.5 mg/dL in serum creatinine or a decrease in glomerular filtration rate (GFR) of 9-15 ml/min during admission has been recognized as being at increased risk for renal dysfunction (8,9). Different biomarkers have been suggested for the diagnosis of renal dysfunction in the setting of heart failure (Table 1.
Biomarkers in heart failure.
| Glomerularfunction | Tubulointerstitialfunction |
|---|---|
| Creatinine | NAG |
| GFR estimation | NGAL |
| BUN | Interleukin 18, |
| Cystatin C | kidneyinjurymolecule 1 |
| Albuminuria | fattyacidbindingprotein |
| Urinaryexosomes |
BUN: blood urea nitrogen; GFR: glomerular filtration rate; NAG: N-Acetyl-β-D-glucosaminidase; NGAL: neutrophilgelatinase-associatedlipocalin.
Classically, the occurrence of renal dysfunction in patients with heart failure has been attributed to a low-flow state in which the decline in glomerular filtration rate is directly determined by reduced cardiac output. Some indirect evidence supports this theory, especially the occurrence of neurohumoral phenomena in patients with heart failure that are precipitated by renal hypoperfusion; in addition, clinical experience has indicated that interventions directed towards increasing cardiac output often restore organ perfusion, as indicated by clinical parameters such as urine output and mental status. Clinically, worsening renal function typically occurs within days of hospitalization, which suggests a direct causative effect of the hemodynamic derangement observed when initiating treatment for acute decompensated heart failure (ADHF), and patients with progressive pump failure or cardiogenic shock often develop progressive renal impairment that is reversible with advanced support.
However, data from clinical trials have challenged this rationale. The ESCAPE trial tested the influence of a pulmonary artery catheter–guided therapy in individuals with acute decompensated heart failure and found no correlation between the baseline renal function and cardiac index (10). Furthermore, an improvement in the cardiac index did not result in improved renal function. This notion is additionally supported by other investigations that showed that an improved cardiac index or decreased pulmonary capillary wedge pressure failed to predict improvement in renal function (11). Data from the ADHERE registry indicated that most patients with acute decompensated heart failure present with elevated, rather than low, blood pressure (12) and that the incidence of worsening renal function is similar among patients with reduced or preserved systolic function (13). Given these findings, other mechanisms have been proposed.
An alternative explanation could involve a redistribution of blood from arterial to venous circulation that leads to an effective reduction in renal blood flow. The resulting reduction in arterial effective volume can result in increased sodium and water absorption through stimulation of the sympathetic nervous system, the renin-angiotensin-aldosterone system and vasopressin secretion to preserve renal perfusion and the renal filtration fraction. However, such mechanisms are often difficult to demonstrate in individual patients at the bedside.
Modifications of transrenal perfusion pressure have increasingly received attention. Transrenal perfusion pressure is calculated as the mean arterial pressure minus the central venous pressure; therefore, for a patient with volume overload and heart failure, the combination of increased central venous pressure with low systemic pressure may lead to a severe compromise of the net renal perfusion pressure. Systemic pressure may be reduced by a decrease in cardiac output and hypotension; additionally, drugs currently used to treat heart failure patients may potentiate these effects. For example, diuretics may cause hypovolemia, and drugs that inhibit the angiotensin system may induce vasodilation of the efferent circulation and reduction in the glomerular perfusion pressure. Because the kidney is an encapsulated organ, when renal congestion occurs, the increased venous pressure may distend the venules surrounding the distal ends of the tubules, which can obliterate the lumen of the tubule (14). Other studies have simply suggested that the increased central venous pressure could be transmitted back to the renal veins, thereby causing an increase in the renal interstitial pressure (15,16). This possibility is reinforced by the observation that intrarenal and systemic angiotensin II concentrations increase with increasing renal venous pressure (17,18). Elevation of renal venous pressure from the extrinsic compression of the veins has also been suggested as a mechanism contributing to reduced renal function in patients with ascites (19). Non-hemodynamic mechanisms are also involved. Hyperactivation of the neurohumoral and inflammatory axes has been associated with progression or renal and cardiac dysfunction. The activation of RAAS and SNS induces increased afterload, myocardial oxygen consumption, sodium retention and ventricular remodeling and is associated with worse outcomes among patients with heart failure. Accordingly, the activation of these systems may also contribute to the acceleration of renal disease, as indicated by the positive effects of RAAS blockade on renal function in patients with hypertension and diabetes. However, these effects are likely mediated by endothelial dysfunction, oxidative stress, fibrosis and inflammation (20).
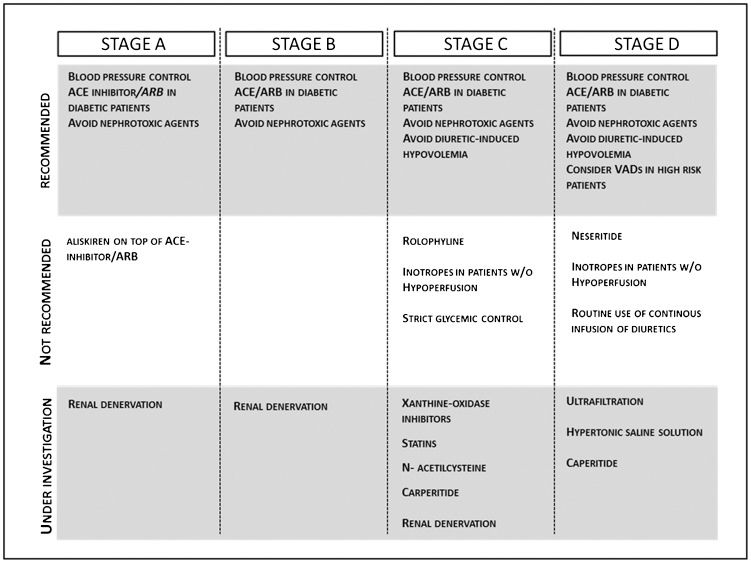
Preventive measuresDespite the recognition of the epidemiological and prognostic relevance of renal dysfunction in patients with decompensated heart failure and all of the efforts directed to the discovery of novel and more accurate methods for its diagnosis, few specific interventions are currently available for the prevention of renal dysfunction in this setting. Most of bedside protocols are based on experimental data or on data derived from other clinical conditions. We will briefly review the general approaches currently available and some specific interventions currently proposed or under investigation, with a focus on the concept that heart failure is a progressive condition and that interventions should be guided by disease stage, patient clinical presentation and the presence of co-morbidities (Figure 2.
General approachAs discussed above, extreme variations in volume can reduce the GFR. In hypovolemia, GFR can be reduced by the reduction of effective plasmatic volume and renal hypoperfusion. In contrast, hypervolemic patients may have impaired renal function because of renal venous congestion, reduced renal perfusion pressure or renal compression. Therefore, intense and sudden variations in the plasmatic volume of patients with decompensated heart failure should be strongly avoided. Thus, excessive diuretic therapy has traditionally been seen as deleterious, and in fact, reduced volume has been described in patients with chronic heart failure under diuretic therapy. However, transient elevations in creatinine (possibly associated with modifications of plasmatic volume and blood pressure) have been considered as common in patients with decompensated heart failure and thus considered to not carry the same prognostic significance as persistent elevations, especially in patients with predominant right ventricular failure who show improvements in congestive phenomena (21). Additionally, other interventions currently considered to be standard for the treatment of acute decompensated heart failure may have deleterious effects on renal function. Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers can decrease filtration pressure within the glomerulus by inducing systemic hypotension and predominant vasodilation of the glomerular efferent arteriole. Although ACE inhibitors and ARBs remain a mainstay for the treatment of patients with chronic heart failure, their utility during episodes of decompensation has not been clearly demonstrated, and current guidelines do not support the interruption of therapy with ACE inhibitors and ARBs during episodes of acute decompensated heart failure. The influence of beta-blockers on renal function is much less understood. A small study suggested that the initiation of beta-blocker therapy was associated with preserved renal function in heart failure patients with a lower baseline GFR but not in those with a higher baseline GFR (22), although these results have yet to be confirmed. Finally, small retrospective studies have suggested that spironolactone may increase creatinine levels (23,24) and should be used cautiously in patients with reduced GFR.
During episodes of acute decompensation, invasive interventions are often required, and clinicians should be aware of the possibility of contrast-induced nephropathy (CIN). Recommendations for the prevention of CIN have been published (25), and additional care should be taken to avoid congestive phenomena during pre-procedure hydration. Frequently, a dose reduction or cessation of the diuretic therapy is performed in place of active intravenous hydration. As generally recommended for patients with chronic heart failure, nonsteroidal anti-inflammatory drugs (NSAIDs) should not be used during an episode of acute decompensation, as NSAIDs induce decreases in prostaglandin E2, which leads to increased sodium and water reabsorption that can result in weight gain and edema. As noted, in individuals with decreased circulating volume, vasodilatory prostaglandins are produced by the kidneys to offset other vasoconstricting autacoids. In a clinical setting in which the renal blood flow depends on prostaglandin synthesis, NSAIDs can significantly decrease renal blood flow, with resultant acute renal failure (26).
Specific interventionsStatinsData from studies in patients with chronic kidney disease suggest that statins may preserve renal function (27). An analysis of the CARE (28) study demonstrated a significant difference in the rate of decline with pravastatin in individuals with severe CKD at baseline relative to the placebo. In the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) study (29), the effect of atorvastatin administered at a dose 10 to 80 mg/day on renal function was compared with the “usual care” in previously untreated dyslipidemic patients with coronary heart disease. At the end of the study, creatinine clearance had increased by 12% in the atorvastatin group and 4.9% in the “usual care” patients. In a subanalysis of the Treating to New Targets (TNT) study (30), the effect of intensive lipid reduction on renal function using 10 mg versus 80 mg of atorvastatin in patients with coronary heart disease was evaluated. The expected 5-year decline in renal function was not observed in this study, and the estimated GFR improved in both TNT treatment groups but was significantly greater with atorvastatin at 80 mg than at 10 mg, which suggests a dose-related benefit. Additionally, a meta-analysis of 13 small prospective, controlled trials examining the effect of statins on renal function showed that treatment with statins reduced the rate of decline in the GFR with a possible trend towards proteinuria reduction (2,31). However, some studies with rosuvastatin indicate a possible negative effect on renal function and proteinuria. The GISSI-HF trial (32) and the CORONA trial (33) tested the effects of statin therapy in patients with chronic heart failure and did not demonstrate any influence on the hard end-points. Therefore, even if statins have a positive influence on the renal function of patients with heart failure, an effect on the overall prognosis is unlikely.
Adenosine A1 receptor antagonistsStimulation of the adenosine A1 receptor on the glomerular afferent arteriole reduces renal blood flow and the glomerular filtration rate (GFR), and also increases sodium and water reabsorption through the stimulation of receptors on the proximal tubules. Adenosine receptor antagonists, such as aminophylline and theophylline, have been used in heart failure patients, but arrhythmic effects have hampered their use. Recently, the PROTECT trial tested the effects of rolofylline as a renal protective strategy in 2,033 patients with acute heart failure, and the results indicated no effect of rolofylline in this setting (34).
Xanthine oxidase inhibitorsData derived from experimental and clinical models have suggested that xanthine oxidase inhibitors have positive metabolic effects in the failing myocardium that provide increases in energy delivered by adenosine triphosphate (35). In a small study of patients with ischemic cardiomyopathy, a single intravenous dose of 400 mg of oxypurinol led to a reduction in end-systolic volume and an increased left ventricular ejection fraction relative to the placebo. In patients with chronic kidney disease (36), the use of allopurinol has been associated with a reduction in cardiovascular events and increases in the GFR (37,38). However, the effects of allopurinol for renal protection in patients with heart failure are still under discussion. Xanthine oxidase-derived reactive oxygen species accumulation leads to nitric oxide scavenging and endothelial dysfunction but also to various other detrimental metabolic, functional and immunologic effects. Novel data are emerging to suggest direct effects of uric acid on immune stimulation and metabolic interference (39).
Renin inhibitorsDespite the current availability of medical treatments for heart failure, the residual rates of hospitalization and death remain high. One putative explanation for these high rates is insufficient RAAS inhibition produced by the triggering of counterregulatory mechanisms of renin release by single-site RAS blockade. Therefore, the use of a specific inhibitor of human renin, such as aliskiren, has been proposed. Aliskiren was tested in addition to ACE inhibitors or ARBs in patients with diabetes mellitus and renal dysfunction; however, an increase in adverse events (increased incidence of renal complications, hyperkalemia, hypotension and non-fatal strokes) and no apparent benefits among the patients randomized to aliskiren prompted the data safety and monitoring board for the study to recommend its termination. Subsequently, the ASPIRE study reported a significant increase in the risk of renal failure, hypotension and hyperkalemia with the combination of aliskiren and an ACE inhibitor or ARB in high-risk post-myocardial infarction patients (40). Ongoing trials will evaluate the effects of aliskiren on the prognosis, ventricular function and renal function in the setting of chronic (41) and acute heart failure (42).
Renal denervationHyperactivation of the SNS is involved in the pathophysiology of hypertension, renal insufficiency and heart failure. Devices have been developed for ablation of the renal sympathetic nerves by a radiofrequency-emitting catheter inserted percutaneously into the femoral artery in the groin and consecutively advanced to lie in the lumen of each renal artery. Sympathetic nerves enter the human kidneys through the walls of the renal arteries and are thus within reach of the ablative energy. This type of intervention has been reported to be successful in patients with resistant hypertension (43). Renal function, as assessed by serum creatinine, eGFR and cystatin C concentrations, was unchanged from the baseline in both groups at 6 months. Several ongoing clinical trials are investigating the safety and efficacy of renal denervation in patients with CHF, and its application as a preventive measure for renal dysfunction remains speculative (44).
Low-dose dopamineThe infusion of low-dose dopamine (4-5 mg kg-1min-1) has been shown to induce renal vasodilation and natriuresis mediated by the stimulation of the dopamine alpha-1 and alpha-2 receptors in the proximal tubule, the thick ascending loop of Henle and the cortical collecting ducts (45). In addition, putative renoprotective effects of dopamine have also been suggested in various clinical settings. In a meta-analysis of 61 trials that randomly assigned 3,359 patients at risk for acute renal failure to receive low-dose dopamine or placebo, dopamine was associated with a 24% increase in urine output, 4% relative decrease in serum creatinine level and 6% relative increase in measured creatinine clearance (46). In patients with systolic heart failure, low-dose dopamine has also been demonstrated to increase renal blood flow and the GFR. In a clinical trial with 60 patients with acute decompensated heart failure, the combination of infused low-dose furosemide and low-dose dopamine was suggested to be as effective as a high-dose furosemide infusion in terms of the clinical and diuretic response and to be associated with a significantly lower rate of worsening renal function, which suggests a renoprotective effect in this patient population (47). The long-term effects of this intervention in a larger population of patients are yet to be determined.
DiureticsDespite the scarce evidence based on prospective data and clinical trials, diuretics have long been a mainstay in the treatment of episodes of acute decompensated heart failure in the presence of congestive phenomena. Concerns regarding diuretic use involve ototoxicity, hypocalcemia and diuretic-induced hypovolemia; these conditions become particularly important in the setting of ADHF because of the presence of hypotension, right ventricular failure and elevated intra-abdominal pressure as well as the use of high diuretic doses. Thus, a renoprotective strategy could potentially be derived from the continuous infusion of smaller doses of a diuretic. Different diuretic strategies were tested in a clinical trial (48) that assigned patients with acute decompensated heart failure to receive furosemide administered intravenously in a bolus every 12 hours or by continuous infusion and at a low or high dose; the authors concluded that there were no significant differences in the change in renal function when diuretic therapy was administered by bolus compared with continuous infusion or at a high dose compared with a low dose.
Human recombinant B-type natriuretic peptideNesiritide is a systemic pulmonary vasodilator that is capable of promoting natriuresis and diuresis and of inhibiting the RAAS. Initial experiments in patients without heart failure and outpatients with stable heart failure produced conflicting results regarding the effect of nesiritide on the GFR, with some studies suggesting an improvement in the GFR and others showing no impact (49,50,51). Later, a meta-analysis found that treatment with nesiritide was associated with worsened renal function (52). A clinical trial with 75 patients with acute decompensated heart failure showed that nesiritide had no impact on renal function (53). Recently, a larger clinical trial with 7,141 patients confirmed that nesiritide had a neutral effect on renal function but was associated with an increase in the rate of hypotension (54). These findings do not warrant the use of nesiritide as a renoprotective measure in patients with ADHF. Other similar molecules, such as alpha-human atrial natriuretic peptide (carperitide), are currently under investigation.
Calcium sensitizersThis class of drugs includes inotropic agents, such as levosimendan, that also produce arterial and venous vasodilation through the activation of ATP-sensitive potassium channels. Because central venous pressure is an independent predictor of the GFR in patients with heart failure, levosimendan has been proposed as an agent that may be associated with improved renal function (55). Thus, levosimendan infusion has been described to improve the GFR more than infusion of dobutamine, even though both treatment regimens were associated with an increase in urine output (56). Another study suggested a sustained improvement in renal function up to 3 months after infusion in patients with advanced heart failure eligible for heart transplantation (57). In the LIDO trial, levosimendan showed greater improvement of serum creatinine levels than dobutamine (58). However, the use of levosimendan in patients with ADHF has been hampered by the results of more recent clinical trials: in SURVIVE (59), the primary endpoint of total mortality was not different between levosimendan and dobutamine; in REVIVE-II (60), the combined clinical endpoint including mortality and symptoms did indicate a benefit of levosimendan over a placebo. Even if the putative beneficial effects of levosimendan on renal function are confirmed, the positive effects on renal blood flow may be offset by the drop in blood pressure and risk of arrhythmia.
UltrafiltrationUltrafiltration (UF) has been proposed as an alternative to diuretics to obtain faster relief of pulmonary/systemic congestion. This method consists of an extracorporeal circuit through which blood is pumped from a venous access into the filter, to be then returned into the patient (61). During IU, water crosses the semipermeable membrane in the filter by ultrafiltration, which is a process driven by the hydrostatic pressure difference across the filter membrane. Small solutes that pass through the ultrafiltration membrane, such as electrolyte, are removed concurrently. Thus, UF offers isotonic removal and maintains the plasma concentration of low-molecular-weight solutes such as sodium and other small solutes. Most of the expected clinical benefit is provided by fluid removal and improvement of pulmonary/systemic congestion; however, neither significant correction of hyponatremia, azotemia, hypo/hyperkalemia or metabolic acidosis/alkalosis, nor a significant removal of high-molecular-weight substances (such as myocardial-depressant factors and cytokines) can be expected.
The UNLOAD trial (62) compared UF with diuretic therapy in patients with ADHF; UF produced greater weight loss and a more negative fluid balance and was associated with a reduced readmission rate. However, patients with hypotension, hemodynamic instability or under vasoactive inotropes were excluded; additionally, no improvement in neurohormonal activation accompanied the fluid removal and weight change. Data on the effects of UF on renal function in heart failure are lacking; small observational studies showed no changes in serum creatinine when UF was compared to diuretics in patients with chronic heart failure (63), and in a small case series, 45% of patients underwent hemodialysis during the same or a subsequent hospitalization after UF (64). Thus far, there is no indication that the use of UF in patients with ADHF may prevent the occurrence of renal dysfunction.
Arginine vasopressin receptor antagonistsTolvaptan was compared with a placebo in a randomized, double-blind trial involving 254 patients with chronic heart failure. When added to standard heart failure therapy, tolvaptan was associated with significant weight reduction secondary to fluid loss relative to the placebo. Among 70 patients with hyponatremia, tolvaptan was associated with improved and often normalized serum sodium concentrations. The Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist in Congestive Heart Failure trial (ACTIV in CHF) was a multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-ranging phase II feasibility trial that compared three once-daily doses of tolvaptan with a placebo in patients with worsening heart failure symptoms. Sixty-eight patients (21.3%) had hyponatremia at randomization. All doses of tolvaptan significantly reduced body weight 24 hours after the first dose, with significant increases in urine volume relative to the placebo. Significant hemodynamic changes and significant differences in the 60-day rehospitalization rate, unscheduled visits for heart failure and deaths were not noted between patients treated with tolvaptan and those treated with the placebo. These preliminary findings in patients with heart failure led to the large, multicenter, international Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST). This trial was a combination of three studies: two were identically designed to investigate short-term effects on global clinical status and symptoms in patients with heart failure, and the third study was designed to analyze all randomized patients to evaluate outcomes, including time to all-cause mortality and time to first cardiovascular mortality or hospitalization for heart failure. EVEREST was a randomized, double-blind, placebo-controlled study that enrolled 4,133 hospitalized patients with symptomatic left ventricular systolic dysfunction (left ventricular ejection fraction ≤40%). The patients were randomized to 30 mg of tolvaptan or a placebo once a day for 60 days in addition to their standard heart failure regimen. The results indicated a significant improvement in patient-assessed dyspnea on day 1 in the tolvaptan group relative to the group receiving the placebo. This improvement in dyspnea could be related to the significant reduction in body weight secondary to aquaresis recorded on day 1 in patients receiving tolvaptan relative to the patients receiving the placebo. Overall, tolvaptan had no effect on all-cause mortality. Similarly, no significant difference in the composite measure of death from cardiovascular causes or hospitalization for heart failure was detected between the tolvaptan and placebo groups (65,66,67).
Hypertonic saline solutionHypertonic saline solutions have been studied in different forms of cardiovascular collapse since 1917 (68), and data from experimental shock models demonstrate that the infusion of 7.5% NaCl produces vasodilatation and increased regional blood flow to the coronary (69), renal (70), intestinal and skeletal muscle (71) circulation. Additionally, hypertonic saline improves renal function and myocardial contractility, probably because of a direct cardiac inotropic effect induced by hypertonicity (72,73). In patients, the infusion of 7.5% NaCl has been successfully used in cardiogenic shock arising from right ventricular infarction (74). Small volumes of saline solutions have also been tested in patients with heart failure (75,76), and most studies have focused on the aspects of safety and effectiveness. A randomized trial reported, as a secondary finding in a selective population of patients highly resistant to diuretics, that the infusion of saline solutions with different tonicities was associated with lower creatinine levels (77). However, few previous studies specifically examined the effects of a saline solution on renal function in patients with decompensated heart failure. Recently, hypertonic saline solution administration was reported to attenuate heart failure-induced kidney dysfunction as indicated by improvement in the renal glomerular filtration rate (as measured by serum cystatin C and creatine levels) and tubular function (as measured by the expression of renal tubular proteins aquaporin 2, NHE3 and urea transporter 1). These data indicate that hypertonic saline is a promising low-cost therapeutic strategy in patients with decompensated heart failure that warrants further investigation.
CONCLUSIONSDespite recent improvements in the understanding of pathophysiological phenomena and in diagnostic tools, therapies targeted towards the prevention of renal dysfunction in patients with heart failure are still lacking. Interventions aimed at pivotal pathophysiological points and administered early during the course of the disease may provide better results. However, prospective data in larger populations for most of the new, currently available strategies are still needed.
AUTHOR CONTRIBUTIONSIssa VS wrote the manuscript. Andrade L and Bocchi EA revised and corrected the text.
No potential conflict of interest was reported.