To analyze the preoperative plasma antigenic concentration and activity of von Willebrand factor and its main cleaving protease ADAMTS-13 in pediatric patients with cyanotic congenital heart disease undergoing surgical treatment and investigate possible correlations with postoperative bleeding.
METHODSPlasma antigenic concentrations (von Willebrand factor:Ag and ADAMTS-13:Ag) were measured using enzyme-linked immunoassays. Collagen-binding assays were developed to measure biological activities (von Willebrand factor:collagen binding and ADAMTS-13 activity). The multimeric structure of von Willebrand factor was analyzed using Western immunoblotting. Demographic, diagnostic, and general and specific laboratory data and surgery-related variables were subjected to univariate, bivariate, and multivariate analysis for the prediction of postoperative bleeding.
RESULTSForty-eight patients were enrolled, with ages ranging from 9 months to 7.6 years (median 2.5 years). The plasma concentrations of von Willebrand factor:Ag and ADAMTS-13:Ag were decreased by 65 and 82%, respectively, in the patients compared with the controls (p<0.001). An increased density of low-molecular-weight fractions of von Willebrand factor, which are suggestive of proteolytic degradation (p = 0.0081), was associated with decreased ADAMTS-13 activity, which was likely due to ADAMTS-13 consumption (71% of controls, p = 0.0029) and decreased von Willebrand factor:collagen binding (76% of controls, p = 0.0004). Significant postoperative bleeding occurred in 13 patients. The preoperative ADAMTS-13 activity of <64.6% (mean level for the group), preoperative activated partial thromboplastin time, and the need for cardiopulmonary bypass were characterized as independent risk factors for postoperative bleeding, with respective hazard ratios of 22.35 (95% CI 1.69 to 294.79), 1.096 (95% CI 1.016 to 1.183), and 37.43 (95% CI 1.79 to 782.73).
CONCLUSIONLow plasma ADAMTS-13 activity is a risk factor for postoperative bleeding in children with cyanotic congenital heart disease, particularly in children undergoing cardiopulmonary bypass.
ADAMTS-13 (adesintegrin and metalloprotease with thrombospondin type 1 motif) is a member of a family of zinc metalloproteases and is primarily synthesized in hepatic stellate cells (1), although other sources have been identified. The principal function of ADAMTS-13 is the physiological (limited) cleavage of von Willebrand factor (VWF). Mutations of the ADAMTS-13 gene are associated with the presence of extra-large VWF multimers in plasma, which is the molecular basis of congenital thrombotic thrombocytopenic purpura (2). Abnormal circulating levels of VWF and ADAMTS-13 have been reported in numerous acquired disorders, including cardiovascular diseases (3,4). Increased plasma VWF antigen (VWF:Ag) and/or decreased ADAMTS-13 activity are generally associated with poor outcomes in these conditions (5,6).
Changes in ADAMTS-13 activity and VWF have been reported in patients undergoing cardiac surgery (7) but have not been investigated in the pediatric population. In patients with cyanotic congenital heart disease (CCHD), abnormalities in coagulation parameters and platelet function are common. These abnormalities tend to be more pronounced in the perioperative period, particularly in subjects undergoing cardiopulmonary bypass (8–10). We designed the present study to investigate preoperative abnormalities in circulating ADAMTS-13 and VWF in children with CCHD and possible correlations with postoperative bleeding.
METHODSPatients and general data recordedPediatric patients with CCHD from the Department of Pediatric Cardiology and Adult Congenital Heart Disease, Heart Institute, Faculdade Medicina da Universidade de São Paulo, São Paulo, Brazil, were evaluated preoperatively on a hospital basis and consecutively included in the study (first operation or reoperation). Neonates, patients under intensive care, and patients undergoing emergency cardiac surgery were not included. Recorded parameters included demographics and general laboratory data, the principal diagnosis (as established by Doppler-echocardiography and, in some cases, angiography), the type of surgery, the need for cardiopulmonary bypass and its duration, and relevant postoperative events (e.g., infection, sepsis, bleeding, and death). Relevant postoperative bleeding was defined as 10 mL/Kg/hour during the first hour and 5 mL/Kg/hour after the first hour, requiring a blood transfusion. The study protocol was approved by the Scientific and Ethics Committee of the Heart Institute and the Hospital das Clinicas da Faculdade de Medicina da Universidade de São Paulo, CAPPESq #7106. Written informed consent was required for patient inclusion.
Biochemical determinationsOne day before the surgery, peripheral venous blood was collected in 1:10 volumes 3.2% sodium citrate for analysis of plasma VWF:Ag, VWF biological activity (VWF:CB), ADAMTS-13 antigenic concentration (ADAMTS-13:Ag), and ADAMTS-13 activity. Protease inhibitors were added to analyze the VWF multimeric composition (11). Plasma VWF:Ag and ADAMTS-13:Ag were determined using enzyme-linked immunosorbent assays (Diagnostica Stago, Asnières, France, and Technoclone, Vienna, Austria, respectively). The results were expressed as U/dL and μg/mL, respectively. Plasma VWF:CB was interpreted as the ability of VWF to bind to collagen (12). An in-house enzyme immunoassay was developed using a peroxidase-conjugated rabbit antihuman VWF polyclonal antibody (Dako Corporation, Carpinteria, CA, USA). The results were expressed as percent activity. The ADAMTS-13 activity was determined using a previously described collagen-binding assay (13). Briefly, diluted plasma samples were added to plate wells precoated with collagen (Vitrogen, Cohesion Corp., Palo Alto, CA, USA) with or without previous dialysis against 1.5 M urea. The binding of VWF to collagen was measured using a peroxidase-labeled rabbit antihuman VWF antibody (Dako Corporation). The ADAMTS-13 activity calculation compared the VWF binding after dialysis (residual) with the binding in the sample not subjected to dialysis. The results were expressed as percent activity. Proteolysis of VWF multimers was analyzed using Western immunoblotting as previously described (11). The results were expressed as the density of VWF low-molecular-weight fractions relative to the density of all the fractions (laser densitometry). In all the determinations, the results were compared with a control group of nine healthy children within the same age range.
Statistical analysisThe results are presented as the mean and standard deviation or median and range. Comparisons between groups were performed using the Student's t-test, Mann-Whitney test, or Kruskal-Wallis test. The association between numeric variables was tested using linear regression and correlation. Logistic regression models were constructed to investigate possible predictors of postoperative bleeding. Once a variable was selected as a potential candidate based on univariate analysis, bivariate analysis was used to test for possible confounders. A confounder was defined as a second variable causing a significant reduction in the hazard ratio associated with the variable under investigation. Multivariate analysis was conducted by including all the variables with a p-value of <0.10 in the univariate and bivariate analyses. A reduced multivariate model was developed using only the variables with a p-value of <0.10 in the first step of the multivariate analysis. In the final analyses, 0.05 was adopted as the significance level.
RESULTSDemographics, diagnosis, and general laboratory dataThe study group consisted of 48 pediatric patients (24 males, aged 9 months to 7.6 years, median age 2.5 years, body weight 11.9±3.7 Kg) with tetralogy of Fallot (N = 11), pulmonary atresia with a ventricular septal defect (N = 14), or a univentricular heart (N = 23). Patients exhibited decreased systemic oxygen saturation compared with the controls (median 80% (range: 53 to 91%) and median 98% (range: 95 to 99%), respectively, p<0.0001), increased hematocrit levels (47±7 and 37±3%, respectively, p<0.0001), increased hemoglobin levels (15.9±2.3 and 12.7±1.2 g/dL, respectively, p<0.0001), and normal platelet counts (313±114×109 and 301±56×109 platelets/L, respectively, p = NS). Blood coagulation test results were within the normal range (activated partial thromboplastin time (APTT) normalized ratio of 1.11±0.13 and prothrombin time international normalized ratio of 1.12±0.11).
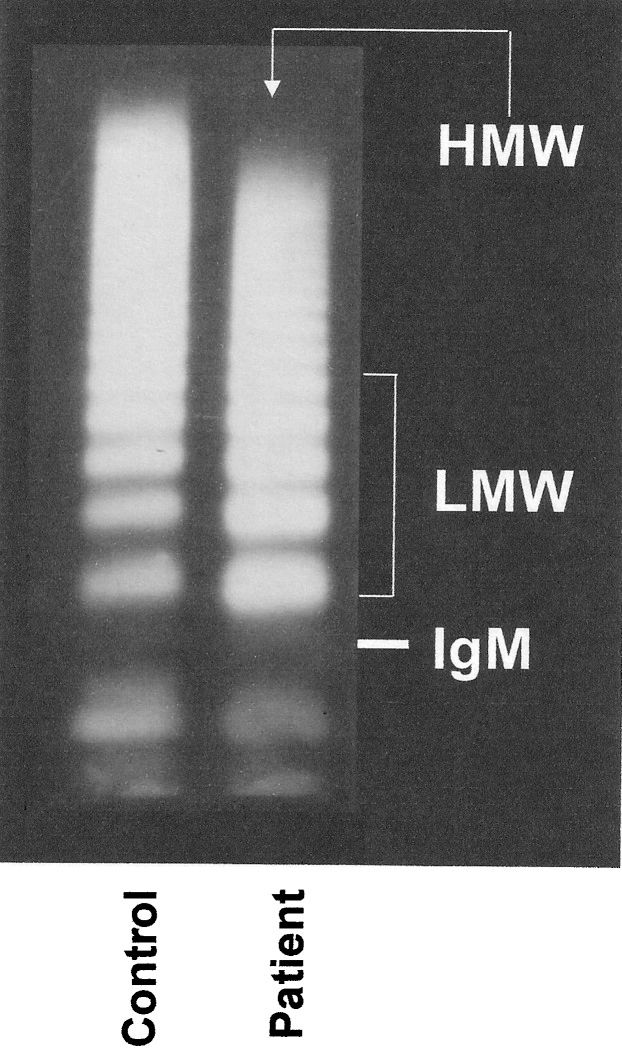
Specific laboratory determinationsThe preoperative VWF:Ag levels were decreased in the patients (Table 1) compared with the controls; the lowest levels were observed in subjects with pulmonary atresia, followed by those with tetralogy of Fallot and a univentricular heart (69, 73, and 85 U/dL, respectively, p = 0.0072). A proteolytic pattern of VWF protein (multimers) was observed (Table 1) and Figure 1, which was associated with decreased VWF:CB and decreased ADAMTS-13 (both antigen and activity, which was likely due to enzyme consumption) (Table 1). The lowest levels of VWF:CB were observed in subjects with the lowest levels of ADAMTS-13 activity (r = 0.60, p<0.0001, Figure 2. The ratio of VWF:Ag/ADAMTS-13 activity did not change because both values were reduced. Except for VWF:Ag, no differences were detected between the diagnostic groups. The only association with general laboratory parameters was a positive correlation between the ADAMTS-13:Ag and the systemic oxygen saturation (r = 0.44, p = 0.002).
Preoperative plasma von Willebrand factor and ADAMTS-13.
| Patients | Controls | p-value | |
|---|---|---|---|
| VWF:Ag (U/dL) | 73 (51-159) | 113 (105-129) | <0.0001 |
| VWF:CB (%) | 48±21 | 63±9 | 0.0004 |
| ADAMTS-13:Ag (μg/mL) | 0.91±0.29 | 1.11±0.09 | 0.0002 |
| ADAMTS-13 activity (%) | 65±24 | 91±24 | 0.0029 |
| VWF:Ag/ADAMTS-13 activity | 1.17 (0.62-4.84) | 1.13 (1.1-1.69) | 0.7427 |
| VWF multimers | 0.44 (0.11-0.56) | 0.36 (0.25-0.55) | 0.0081 |
ADAMTS-13:Ag, plasma ADAMTS-13 antigenic concentration; VWF:Ag, plasma von Willebrand factor antigenic concentration; VWF:Ag/ADAMTS-13 activity, ratio of von Willebrand factor antigenic concentration to ADAMTS-13 activity; VWF:CB, plasma von Willebrand factor activity expressed as collagen-binding capacity; VWF multimers, ratio of low-molecular-weight multimer density to total multimer density of von Willebrand factor.
Western immunoblotting of the plasma von Willebrand factor multimeric structure. Compared with the controls, children with cyanotic congenital heart diseases exhibited decreased densities of high-molecular-weight (HMW) multimers and increased concentrations of low-molecular-weight (LMW) fractions (5 bands migrating above the IgM position, 950 kDa), which indicates abnormal proteolytic degradation. This pattern was observed in 36 of the 48 patients (p = 0.0081, densitometric analysis shown in Table 1).
Correlation of von Willebrand factor biological activity (VWF:CB) with ADAMTS-13 activity in children with cyanotic congenital heart disease. The dashed lines correspond to mean values in the controls. The lowest levels of ADAMTS-13 activity correlated with the lowest levels of VWF:CB, which indicates the involvement of enzyme consumption in the degradation of biologically active (high-molecular-weight) von Willebrand factor multimers.
The patients were subjected to total repair of tetralogy of Fallot (N = 11) or pulmonary atresia (N = 7), Glenn- (N = 14) or Fontan-type (N = 9) cavopulmonary connection, modified Blalock-Taussig anastomosis (N = 6), or pulmonary artery banding (N = 1). Cardiopulmonary bypass was required in 31 instances (duration of 38 to 250 minutes). Three immediate postoperative deaths associated with low cardiac output and circulatory collapse occurred. Significant postoperative bleeding, as previously defined, occurred in 13 patients. Systemic infection or sepsis did not occur in any of the patients.
Predictors of postoperative bleedingPreoperative clinical and laboratory parameters, including VWF and ADAMTS-13 determinations, were tested for possible relationships with postoperative bleeding. Of the specific biochemical markers, low ADAMTS-13 activity was the only parameter exhibiting a significant association in the univariate analysis. An activity of <64.6% (mean (default) level in the patient group) was associated with a hazard ratio of 11.33 (95% CI 1.33 to 96.81). The bivariate analysis results presented in Table 2 showed that the only potential confounder was VWF:Ag. However, a specific association of VWF:Ag with bleeding could not be demonstrated (univariate and bivariate analyses). Of the other clinical and laboratory parameters, preoperative APTT and cardiopulmonary bypass were also associated with postoperative bleeding, with p<0.05 in the univariate analysis (Table 2), variables tested individually).
Bivariate analysis of preoperative ADAMTS-13 activity as a predictor of postoperative bleeding.
| 95% CI for the | ADAMTS-13 | Second variable | |||||
|---|---|---|---|---|---|---|---|
| Number of | Hazard | hazard ratio | Activity | In the model | Singly | ||
| patients | ratio (∗) | Lower | Upper | p-value | p-value | p-value | |
| Unadjusted (univariate analysis) | 48 | 11.33 | 1.33 | 96.81 | 0.0265 | (‡) | |
| Adjusted (bivariate analysis) | |||||||
| Patient age | 48 | 12.38 | 1.38 | 111.14 | 0.0246 | 0.0616 | 0.0587 (‡) |
| Body weight | 48 | 12.77 | 1.44 | 113.63 | 0.0223 | 0.0717 | 0.0818 (‡) |
| Type of anomaly | 48 | 12.08 | 1.38 | 105.96 | 0.0245 | 0.6762 | 0.9195 |
| Oxygen saturation | 48 | 11.90 | 1.38 | 102.82 | 0.0244 | 0.4810 | 0.6124 |
| Hemoglobin | 48 | 15.27 | 1.58 | 147.35 | 0.0184 | 0.2017 | 0.5086 |
| Platelet count | 48 | 14.85 | 1.59 | 138.77 | 0.0180 | 0.0627 | 0.0963 (‡) |
| Leukocyte count | 48 | 18.72 | 1.88 | 186.79 | 0.0126 | 0.0342 | 0.0588 (‡) |
| PT (INR) | 48 | 11.64 | 1.32 | 102.27 | 0.0269 | 0.0895 | 0.0745 (‡) |
| APTT | 48 | 10.06 | 1.11 | 91.25 | 0.0402 | 0.0526 | 0.0228 (‡) |
| VWF:Ag | 48 | 7.73 | 0.85 | 70.63 | 0.0701 | 0.3115 | 0.0748 |
| VWF:CB | 48 | 9.96 | 1.08 | 91.68 | 0.0424 | 0.6873 | 0.1818 |
| VWF multimers | 48 | 11.41 | 1.31 | 99.43 | 0.0275 | 0.3280 | 0.3000 |
| ADAMTS-13:Ag | 48 | 15.58 | 1.63 | 148.90 | 0.0171 | 0.0814 | 0.1733 |
| VWF:Ag/ADAMTS-13 activity | 48 | 13.85 | 1.50 | 128.16 | 0.0206 | 0.5048 | 0.8113 |
| CBP | 48 | 13.15 | 1.44 | 119.86 | 0.0223 | 0.0289 | 0.0346 (‡) |
| CPB time | 31 | 11.84 | 1.23 | 114.40 | 0.0326 | 0.3727 | 0.2658 |
| Reoperation (†) | 48 | 12.73 | 1.44 | 112.64 | 0.0222 | 0.0963 | 0.1231 |
ADAMTS-13:Ag, plasma ADAMTS-13 antigenic concentration; APTT, activated partial thromboplastin time expressed as a normalized ratio; CPB, cardiopulmonary bypass; PT, prothrombin time expressed as the international normalized ratio (INR); VWF:Ag, plasma von Willebrand factor antigenic concentration; VWF:Ag/ADAMTS-13 activity, ratio of von Willebrand factor antigenic concentration to ADAMTS-13 activity; VWF:CB, plasma von Willebrand factor activity expressed as collagen-binding capacity; VWF multimers, ratio of low-molecular-weight multimer density to total multimer density of von Willebrand factor
(∗) Associated with a preoperative ADAMTS-13 activity below the default (mean) level of 64.6%.
(†) Existing cardiac surgery prior to the current surgery.
(‡) All variables with a p-value of <0.10 are indicated on the right in both univariate and bivariate analyses; these variables were tested using the multivariate model.
According to previously established criteria, eight variables were tested using multivariate analysis (Table 2). The final reduced multivariate model included three predictors of postoperative bleeding, including low preoperative ADAMTS-13 activity (hazard ratio of 22.35 in the final model, 95% CI 1.69 to 294.79), preoperative APTT (hazard ratio associated with an increase of 0.01 in the normalized ratio of 1.096, 95% CI 1.016 to 1.183), and the need for cardiopulmonary bypass (hazard ratio 37.43, 95% CI 1.79 to 782.73). There were no significant associations among the three variables. Significant postoperative bleeding occurred in 40% of the patients with low preoperative ADAMTS-13 activity (versus 6% in the subjects with an ADAMTS-13 activity above the mean/default level, p<0.02), 39% of the patients undergoing ON-pump surgery (versus 6% of the patients who were operated on OFF-pump, p<0.02), and 55% of the patients with both risk factors (versus 7% of the patients with one or no risk factors, p<0.002). The proposed model was used to estimate the likelihood of bleeding:
where:P = probability of postoperative bleeding
x1 = 0 or 1 for the absence or presence, respectively, of cardiopulmonary bypass
x2 = 0 or 1 for the absence or presence, respectively, of ADAMTS-13 activity <64.6%
x3 = activated partial thromboplastin time (normalized ratio)
In the three patients who died postoperatively, the preoperative biochemical parameters were within the range of those in the remaining patients, and the structure of the VWF protein was similar to the structure shown in Figure 1.
DISCUSSIONThe response of endothelial cells to injury involves the release of numerous substances, including VWF. Increased circulating levels of VWF are observed, particularly in acute disorders (5,6), and VWF is considered to be an acute-phase reactant. Several stimuli and conditions have been shown to induce the release of VWF from endothelial Weibel-Palade bodies, including hypoxia, epinephrine, thrombin, fibrin, cytokines, endotoxin, components of the complement system, and reactive oxygen intermediates (14–18). ADAMTS-13 activity should theoretically increase in response to the evaluation of plasma VWF, but in practice, the enzyme activity varies considerably (19). In conditions associated with a marked elevation of plasma VWF:Ag (for example, generalized systemic inflammation), the ADAMTS-13 activity decreases. The decreased activity is likely due to consumption of the enzyme, which is associated with an increase in the VWF:Ag/ADAMTS-13 activity ratio and is a risk factor for complications. In these instances (enzyme depletion), extra-large VWF multimers are detected in circulation (6), which may be associated with a generalized tendency toward thrombosis and coagulopathy.
Overt endothelial cell activation was not likely in our relatively stable patients with chronic CCHD, and the plasma VWF:Ag ratio was not elevated. However, an ongoing process of abnormal VWF proteolytic degradation may have been associated with the increased density of low-molecular-weight VWF species (multimeric analysis) and decreased VWF antigen and activity. Decreased ADAMTS-13 activity could be explained by consumption, although altered enzyme synthesis and/or abnormal degradation cannot be excluded. ADAMTS-13:Ag values correlated directly with systemic oxygen saturation in the study.
The hypothesis that chronic proteolysis of VWF accounts for ADAMTS-13 consumption in children with CCHD is plausible because altered flow conditions (present in nearly all of the included anomalies) have been shown to facilitate the binding of VWF to platelet membrane glycoproteins (particularly glycoprotein Ib), followed by enzymatic degradation (20,21). This mechanism has been proposed to explain the abnormalities in circulating VWF observed in patients with aortic stenosis associated with an increased risk of postoperative bleeding (22,23). We speculate that in children with CCHD, abnormal interactions of VWF with membranes may occur at sites of altered flow conditions (for example, either normal or surgically created systemic-to-pulmonary connections). The lowest VWF:Ag levels in the study were observed in the pulmonary atresia subgroup, in which such connections are generally present. Thus, the link between decreased preoperative ADAMTS-13 activity and postoperative bleeding likely resulted from an ongoing process of pathological VWF cleavage with loss of the largest (high-molecular-weight) multimers, which are the most active multimers in promoting platelet adhesion and aggregation. ADAMTS-13 consumption was suggested by the association of low ADAMTS-13 activity with low VWF biological activity (Table 1 and Figure 2) in the presence of defective VWF multimeric structures (Figure 1). For extra-large VWF multimers to be observed in plasma (not the case in this study), a more dramatic reduction of the ADAMTS-13 activity would likely be necessary (6). The lack of a direct correlation between VWF multimeric abnormalities and postoperative bleeding in our patients is not surprising. Other authors have suggested that the ADAMTS-13 activity and the VWF antigen level are more useful for diagnosing complications than analyzing the VWF multimer alone (6).
After a careful analysis of factors potentially associated with postoperative bleeding, we identified three apparently independent predictors: ADAMTS-13 activity, APTT, and the need for cardiopulmonary bypass. We did not evaluate other abnormalities in coagulation factors and platelet function that may be involved in cardiopulmonary bypass with postoperative bleeding, which may be a limitation of the study. ADAMTS-13 cleaves VWF physiologically, but other enzymes are also involved under pathological conditions. Several enzymes promote VWF proteolysis, including elastase and plasmin (24,25). The activity of these enzymes may be enhanced following cardiopulmonary bypass. Further complementary studies are required to evaluate other enzymes.
In conclusion, we demonstrated that preoperative abnormalities in circulating VWF and ADAMTS-13 may be associated with an increased risk of postoperative bleeding in children with CCHD. Low preoperative ADAMTS-13 activity (e.g., below the mean level for the group) appears to be predictive of significant bleeding. Preoperative APTT and the need for cardiopulmonary bypass were also identified as risk factors. To our knowledge, this is the first study to reveal an association between the activity of the VWF-cleaving protease and the risk of bleeding in patients with CCHD undergoing surgery. Further studies are required to provide a better understanding of the complex nature of perioperative coagulation abnormalities.
AUTHOR CONTRIBUTIONSSoares RP was responsible for the initial intellectual issues, construction of the study protocol, work plan, grant submission, data collection and analysis, blood sampling, laboratory routine, and preparation, review and approval of the manuscript final version. Bydlowski SP was responsible for the initial intellectual issues, construction of the study protocol, work plan, review and approval of the manuscript final version. Jatene MB was responsible for the initial intellectual issues, general patient care, review and approval of the manuscript final verion. Hironaka JF was responsible for the general patient care, data collection, blood sampling and laboratory routine, review and approval of the manuscript final version. Lopes AA is the corresponding author who was responsible for the initial intellectual issues, construction of the study protocol, work plan, grant submission, data analysis, and preparation, review, approval and submission of the manuscript final version.
This work was supported by the Foundation for Research Support of the State of São Paulo (FAPESP, grant # 0559890-9), São Paulo, Brazil, and was performed on a strictly academic basis.
No potential conflict of interest was reported.