The objective of this paper was to characterize the population seen at the dentistry unit of the hematology-oncology service of the Oncology-Hematology Service, Instituto da Criança at the Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo. Oral problems resulting from cancer therapy increase the risk of infection, length of hospital stay, treatment cost and negative impact on the course and prognosis of the disease.
METHOD:Of the 367 medical records of cancer patients seen from November 2007 until December 2008: 186 with a cancer diagnosis and complete clinical data were selected, while 20 with a cancer diagnosis and incomplete records were excluded; 161 medical records with only hematological diagnosis were also excluded. The following characteristics were assessed: ethnicity, gender, age, diagnosis and characteristics of the neoplasm, cancer therapy status and performed dental procedures.
RESULTS:Review of 1,236 visits indicated that 54% (n=100) of the patients had blood cancers, 46% (n=86) had solid tumors and 63% were undergoing anticancer therapy. The proportion of males (52.7%) in the study population was slightly greater. The most common cancer was acute lymphocytic leukemia (32.2%). Cancer occurred more often among those patients aged 5 to 9 years. The most common dental procedures were restorative treatment, preventive treatment and removal of infectious foci.
CONCLUSION:The characteristics of the studied population were similar to those of the general Brazilian and global populations, especially regarding gender and diagnosis distributions. The aim of implementation of the dentistry unit was to maintain good oral health and patients’ quality of life, which is critical to provide oral care and prevent future oral problems.
Established in 2007, the dentistry unit of the Oncology-Hematology service of the Child’s Institute at the Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo (ICr/HCFMUSP), sees patients aged from birth until young adulthood who are undergoing cancer therapy. The unit also provides care to patients with blood diseases, such as congenital or acquired anemia and platelet disorders.
Oral problems resulting from cancer therapy increase the risk of infection, length of hospital stay, treatment cost and negative impact on the course and prognosis of the disease.1
In addition to periodical visits to the ward, the dentistry team focus on preventive actions and dental treatment before, during and after chemo- or radiotherapy. The objective of their work is to prevent infections, maintain oral hygiene and minimize the adverse effects of anticancer therapies.
In addition to these objectives, the unit acts as a reference center for the development of research, such as that reported here. The goal of this retrospective study is to characterize the population treated at a dentistry unit in a pediatric oncology teaching hospital.
MATERIAL AND METHODOf the 367 medical records of dental unit patients seen in the 14-month study period (from November 2007 to December 2008), 186 with a cancer diagnosis and complete clinical data were selected; 20 with a cancer diagnosis and incomplete data were excluded and 161 medical records with only a hematological diagnosis were excluded. The following characteristics were assessed: ethnicity, gender, age, diagnosis, characteristics of the cancer, currently undergoing cancer therapy and dental procedures performed.
RESULTSEvaluation of 1,236 appointments during the study period yielded the following observations:
1. Diagnosis: 54% (n=100) of the patients had hematological malignancies, and 46% (n=86) of the patients had solid tumors. Most of the patients (n=117) (63%) were undergoing cancer therapy. Figure 1 shows the most prevalent types of cancer in the study population. Table 1 shows the distribution of the types of cancer diagnosis identified in the 186 patients. There is a clear prevalence of acute lymphocytic leukemia (32.2%) and leukemia (Table 1).
Diagnoses of the study population.
| Diagnosis | N. of patients | % |
|---|---|---|
| Leukemias (general) | 71 | 38.2 |
| Acute Lymphocytic Leukemia | 60 | 32.2 |
| CNS tumors | 21 | 11.3 |
| Lymphomas | 20 | 10.8 |
| Neuroblastoma | 13 | 6.9 |
| Retinoblastoma | 9 | 4.8 |
| RMS | 9 | 4.8 |
| Wilms’ tumor | 8 | 4.3 |
| Osteosarcoma | 8 | 4.3 |
| Histiocytosis | 6 | 3.2 |
| Lymphoepithelioma | 4 | 2.1 |
| Thyroid cancer | 2 | 1 |
| Nasal angiofibroma | 2 | 1 |
| Mesenchymal tumors | 2 | 1 |
| Ewing’s sarcoma | 6 | 3.2 |
| Other | 8 | 4.3 |
| Total | 186 | 100 |
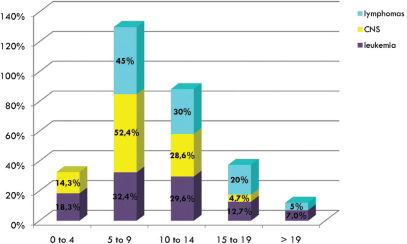
2. Gender and age: A slight increase in males (52.7%) compared to females (47.3%) was observed (Figure 2). The pediatric age groups most commonly affected by cancer are children aged 5 to 8 years, followed by those patients aged 10 to 14 years and finally those patients aged 0 to 4 years (Figure 2). The distribution of cancer prevalence related to gender was also evaluated (Figure 3). Males were more affected compared to females in the most prevalent childhood cancer (leukemias, lymphomas and tumors of the central nervous system). Figure 4 shows that the most common cancers among children aged 5 to 9 years are leukemias, lymphomas and tumors of the central nervous system.
3. Dental procedures: The most common dental procedures among the study population were restorative treatment, preventive procedures (prophylaxis, fluoride and oral hygiene), and removal of infectious foci (Figure 5).
DISCUSSIONThe recently established Dentistry Unit in our Oncology-Hematology Service optimized the care provided to patients and provided better interaction and close cooperation between the dental and medical teams, benefiting the patients and their families.
This specialized unit is essential in Brazilian hospital settings because more than 9,000 new childhood cancers are diagnosed annually in Brazil. Cancer is the third leading cause of death in the 1- to 19-year-old age group in this country, behind accidents and violence.2
The types of cancers that affect children under 15 years of age are distinct from those that affect adults.3 The literature reports that during childhood, acute lymphocytic leukemia (ALL) is the most common malignancy, representing 24% of all childhood malignancies4–6 and 75% of all childhood leukemias.7 The findings of this study are consistent with the data presented in the literature. In our study ALL represents 84.7% of all leukemias and 32.2% of all childhood malignancies.
The 0- to 4-year-old age group is cited as the age group most frequently affected by cancers. In this study, most (56.5%) of the cancer patients were aged 5 to 14 years.
In Brazil, leukemia predominates in the 1- to 4-year-old age group (31.6%), lymphoma is dominant in the 15- to 18-year-old age group (35.6%), and tumors of the central nervous system have a similar prevalence (26%) in all patients under 14 years (1 to 4, 5 to 9 and 10 to 14 years).2 The results of this study show that the most common malignancies in the 5- to 9-year-old age group were leukemias, lymphomas and tumors of the central nervous system. This finding should be further investigated in a more diverse patient population to avoid bias introduced by selection of patients from a pediatric oncology teaching hospital.
Regarding the comparison between genders, this study and other related studies,8 agree that general tumors, leukemias, lymphomas and tumors of the central nervous system are more prevalent in males than in females.
Many authors 9–11 report that the most common complications of pediatric cancer therapy are mucositis, xerostomia, bleeding, dysguesia, widening of the periodontal ligament space and infections (including bacterial, fungal and viral). These complications cause pain and discomfort, require parental narcotic therapy and extended hospitalizations, increase costs, and interfere with the course and prognosis of the neoplasm.12
Dental abnormalities are the most common late complications in children subjected to cancer therapy; among these abnormalities are alterations of shape (microdontia, macrodontia, taurodontia), number (anodontia) and root formation (root shortening and blunting of the roots, root stunting) of the teeth.13–15 Children who undergo head and neck radiotherapy may experience abnormalities in the growth and maturation of the craniofacial skeleton structures,16,17 which can cause severe cosmetic and functional sequelae that require surgical and orthodontic interventions.18
In our study, 63% of the patients were undergoing cancer therapy, and 37% had finished their treatment; therefore, periodic dental follow-ups are essential in both groups to diagnose complications and intervene as early as possible during cancer therapy. Furthermore, orthodontic treatment provided by this unit may be necessary to prevent and treat the craniofacial abnormalities caused by cancer therapy.
Another side effect of chemotherapy is a drop in blood cell count, which is generally seen 5 to 7 days after the beginning of each cycle and usually lasts for 14 days. The period during which the blood cell count is low varies according to the treatment protocol.19
For this reason, the oral cavities of all pediatric patients should be assessed before the beginning of cancer treatment whenever possible to eliminate potential infectious foci and dental infections.20,21 When time is limited for dental care before cancer therapy, treatment priorities should be infections, extractions, periodontal care and sources of irritation.19
During the 14-month study period, the most common dental procedures were restaurative treatment, preventive procedures and removal of infectious foci, given the poor heath of the patients before cancer therapy. The dental procedures and oral care are performed to provide information and prevent future oral complications.
Another treatment that was used quite frequently was low-power laser therapy to minimize one of the most debilitating side effects of cancer therapy: oral mucositis.
Oral mucositis is a debilitating consequence and dose-limiting factor of anticancer therapy. It manifests as a burning sensation in the oral mucosa that can evolve to edema, erythema with formation of ulcers and pseudomembranes. It results in frequent extreme pain that requires dietary changes and parenteral administration of analgesics.22
An efficient treatment for prevention of the onset of mucositis has not been described in the literature. Treatment with a low-power laser has been used in many wound models to promote tissue regeneration23 and to effectively reduce the severity of mucositis in animal24–26 and human27–29 studies. This finding is in agreement with our clinical results.
CONCLUSIONIn conclusion, the studied population presented characteristics that are similar to characteristics of the general Brazilian and world populations, especially regarding gender and diagnosis among cancer patients. In our study population, the establishment of a dentistry unit was essential to provide necessary oral care and prevent future problems, with the aim of maintaining the patients’ oral health and improving quality of life.