Roux-en-Y gastric bypass is a popular and successful operation for the treatment of morbid obesity. However, it greatly restricts ingestion and moderately interferes with absorption of food, thus potentially paving the way for undernutrition, especially during the first year before patients adapt to the new condition. Aiming to document actual dietary intake during this period, a prospective observational study was performed.
METHODSForty consecutive patients were investigated using a 24-hour dietary recall technique every 3 months after surgery for 1 year. Females only were accepted for greater homogeneity of the sample. All received a vitamin and mineral supplement on a daily basis as a postoperative routine. A questionnaire was employed regarding general, nutritional, and gastrointestinal changes as well as consumption of medications. Dietary intake was analyzed after data processing using the Virtual Nutri software package (São Paulo, SP, Brazil).
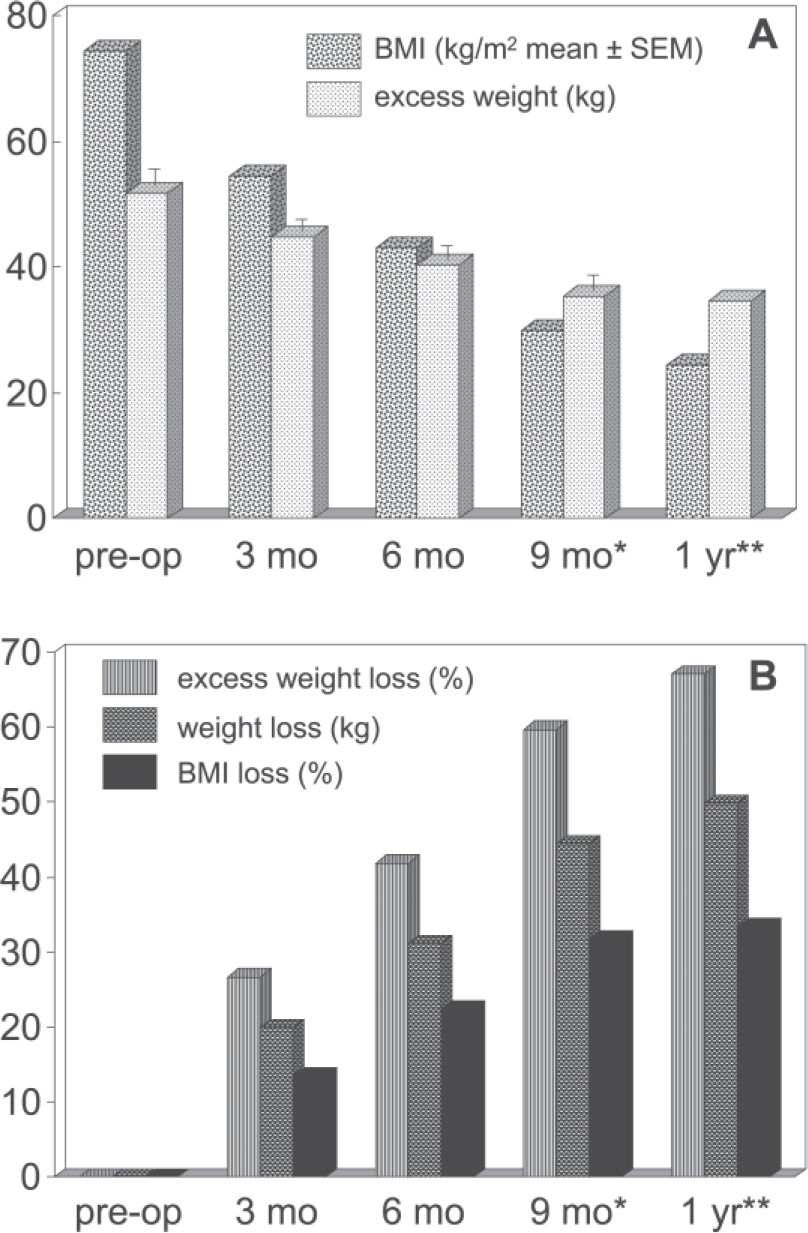
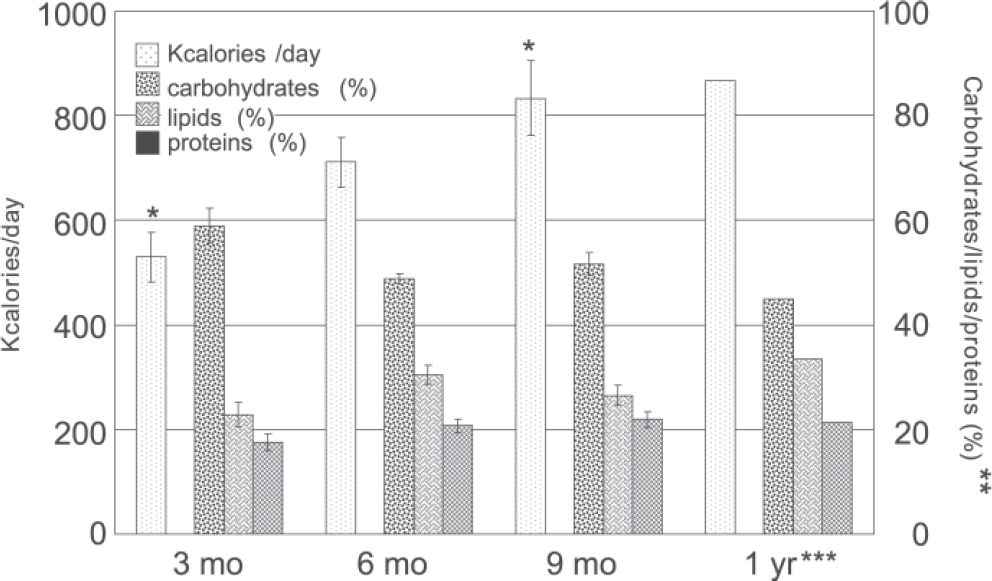
RESULTSThe surgical response was within the expected range, with about 67% excess weight loss at the end of the 1st year, and the same occurred with gastrointestinal symptoms and drug requirements. Daily energy intake on the 4 analyzed occasions was 529.4 ± 47.4, 710.9 ± 47.6, 833.2 ± 72.0, and 866.2 ± 95,1 kcal/day (mean ± SEM); protein intake was increased in the same proportion at 6 and 9 months, but reduced at 12 months. Thus, patients did not meet standard recommendations regarding calories and proteins, even at the end of the 1st year; iron and zinc intake were also inadequate, although deficiencies were probably staved off by the prescribed supplement preparation.
CONCLUSION1) The risk for postoperative undernutrition was evidenced up to 1 year, while spontaneous improvement in food intake was slow and inefficient; 2) Specific protocols should be devised to improve nutrition and health during the postoperative phase until successful dietary adaptation is achieved.
A gastroplastia com anastomose gastrojejunal em Y de Roux é uma operação popular e bem sucedida no tratamento da obesidade grave. Ela restringe seriamente a ingestão e moderadamente a absorção do alimento, potencialmente abrindo caminho para desnutrição especialmente no primeiro ano, antes que o paciente se adapte à nova condição. Com o propósito de documentar a real ingestão neste período, um estudo prospectivo observacional foi executado.
MÉTODOQuarenta pacientes consecutivos foram investigados por recordatório de 24 horas a cada três meses após a operação, até um ano. Apenas mulheres foram arroladas para maior homogeneidade da amostra. Todas receberam diariamente um suplemento vitamínico-mineral, como rotina pós-operatória. Um questionário foi empregado abordando alterações gerais, nutricionais e gastrointestinais assim como consumo de medicamentos. Os ganhos dietéticos foram analisados mediante o programa Virtual Nutri (São Paulo, SP, Brasil).
RESULTADOSA resposta cirúrgica situou-se dentro da faixa esperada, com perda de cerca de 67% do excesso de peso após um ano, e o mesmo ocorreu com sintomas gastrointestinais e necessidades medicamentosas. A quantidade de energia diária nas quatro ocasiões foi de 529,4±47,5, 710,9± 47,7, 833,2± 72,0 e 866,2± 95,1 kcal/dia (média ± erro padrão da média), e o aumento do consumo de proteína foi da mesma proporção nos 6 e 9 meses e com redução em 12 meses. Consequentemente mesmo após um ano as pacientes estavam abaixo das recomendações usuais de calorias e proteínas. A contribuição da dieta no tocante a ferro e zinco também mostrou-se inadequada, embora quadros deficitários tenham provavelmente sido abortados pelo suplemento utilizado.
CONCLUSÕES1) O risco para desnutrição pos-operatória ficou demonstrado até um ano, e a melhora espontânea da ingestão de alimentos revelou-se lenta e ineficiente; 2) Protocolos específicos deveriam ser elaborados visando melhorar a nutrição e a saúde na fase pós-operatória, até que se verifique uma adaptação dietética satisfatória;
Obesity is a multifactorial chronic condition, and excessive food intake is not necessarily the main etiologic mechanism. Therefore, a multidisciplinary approach is always recommended in the management of this disease.1 Nevertheless, the current mainstay of anti-obesity treatment in the case of morbidly obese subjects is drastic surgical reduction of food utilization by means of restrictive, malabsorptive, or mixed interventions.2,3
The most commonly employed operation for treatment of morbid obesity in Brazil and one of the most popular in the world is the Gastroplasty with Roux-en-Y gastric bypass (RYGB), a mixed technique that prevents early food contact with bilio-pancreatic secretions by means of a 100-cm jejunal limb. More importantly, a micro-stomach with just 30 to 50 mL capacity remains functional, since all the remainder of the organ is excluded from digestive transit.4
Reliable long-term weight loss is the desired effect, and indeed most patients achieve reductions of 70% to 80% of the excess weight (actual minus ideal weight), rendering this operation one of the best available and the gold standard of bariatric procedures according to many.5,6 However, serious nutritional deficits after this modality have been reported but are deemed rare,7 and the majority of patients report a substantially improved quality of life in the follow-up period, with a regression of most comorbidities.8
Quantitative food intake has only occasionally been studied in this population, but available findings suggest an adequate pattern following RYGB as well as other predominantly restrictive techniques, thus establishing the basic safety and efficacy of these bariatric maneuvers.3,9,10 Nevertheless, gastrointestinal complaints, especially vomiting but also heartburn and dumping, are not uncommon after this operation, and combined with food intolerances and psychological difficulties could potentially lead to poorer dietary input than currently admitted.
Given these questions, a prospective study was designed aiming to systematically document nutrient intake at 3-month intervals, during the first 12 months after uncomplicated RYGB. A female-only protocol was adopted for greater homogeneity, as this gender represents around 90% of the bariatric candidates of the hospital.1,8
METHODSPopulation: 40 consecutive bariatric patients were periodically assessed during the postoperative period of 1 year regarding ingestion of macronutrients, as well as micronutrients (vitamin A, C, B12, zinc, and iron). Setting: University-affiliated public hospital Inclusion in the study required fulfillment of all of the following: female adult; having undergone elective RYGB, with no previous bariatric intervention, and giving informed consent.
Exclusion criteria were any of the following: sepsis, shock, coma, or multiple organ failure; postoperative fistula, stenosis, or peritonitis; reoperation or rehospitalization for any cause; depression or alcohol or substance addiction; refusal to participate in the study.
Study design: This was a prospective observational cohort study with a 1-year follow-up period.
Clinical procedures: A questionnaire was employed aiming at eliciting demographic information, general and nutritional history, surgical complications, anthropometric findings, gastrointestinal abnormalities, alcohol or substance abuse, and medications. The 24-h dietary recall technique was applied by a single trained dietitian and analyzed using the Virtual Nutri software program (São Paulo, Brazil11), regarding both macronutrients and micronutrients. This software has been validated for Brazilian foods and culinary measures.
Statistical analyses: Values are presented as mean ± SEM. Differences between the various periods were investigated by means of analysis of variance (ANOVA), after normal distribution was confirmed by the Kolmogorov-Smirnov test, with the Tukey post-hoc test. A significance level of 5% (P < 0.05) was adopted.
RESULTSPreoperative findings: The age of the population was 42.5 ± 10.8 years (100% females, as previously indicated), and the initial BMI was 51.9 ± 11.8 kg/m2. Follow-up was 100% at 3 and 6 months, but diminished to 80% (32 subjects) at 9 months, to 32.5% (13 subjects) by 1 year. This last observation is less reliable, and therefore the 12-month values are presented without statistical analysis. The evolution of body weight and body mass indexover the entire interval can be seen in Fig. 1.
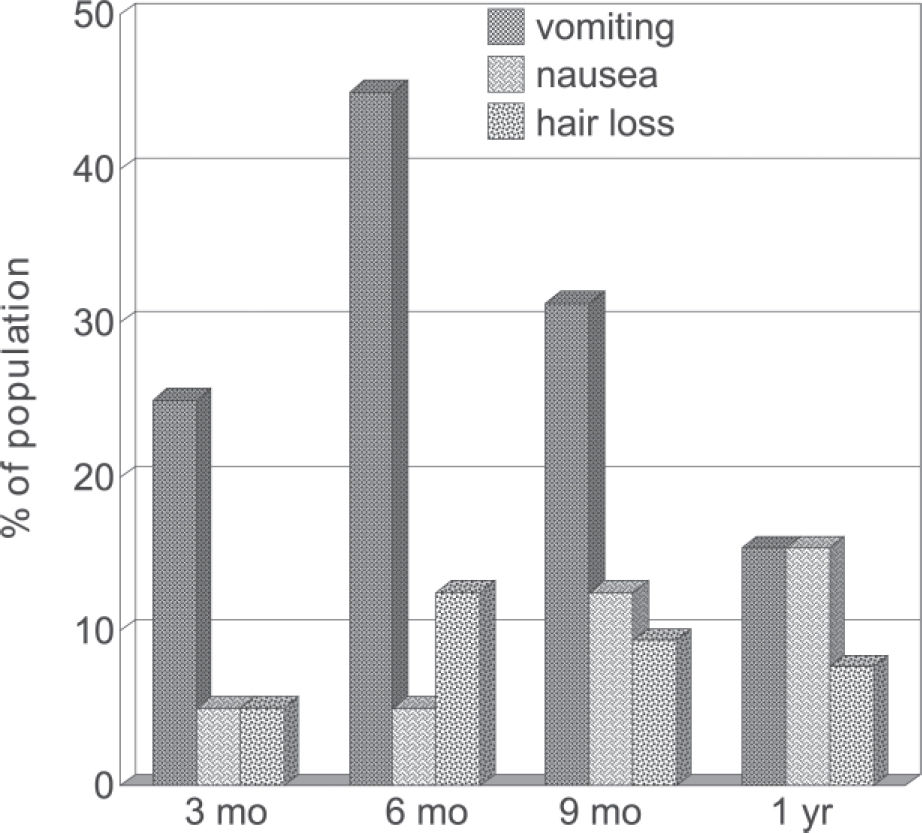
Postoperative gastrointestinal and general symptoms: The most prominent complaints were nausea, vomiting, and hair loss (Figure 2). However, weakness was often observed in the first trimester (12.5%) and to a lesser degree in the ensuing periods; other complaints, such as abdominal pain, dizziness, and general malaise, were reported as well.
Medications: Most patients took different therapeutic drugs, as listed in Table 1. The most frequent pharmacologic categories were vitamins, proton pump inhibitors, anti-hypertensive agents, antibiotics, and antidiabetic products.
Energy intake: The calorie content of the diet started very low and increased slowly; therefore, even after 1 year, patients were still consuming a very restricted regimen. Partition among carbohydrates, protein, and lipids did not reveal statistical differences between the various periods, and percentages were consistent with usual nutritional patterns (Figure 3).
Micronutrients: The mean vitamin intake was acceptable, but contribution of the diet (multivitamin supplement not included) was insufficient to supply as per the international recommendations often used in Brazil (DRI 200012) with respect to iron and zinc at nearly every point of measurement (Table 2).
Vitamin and mineral content of the diet (mean ± SEM)*
| Category | 3 months*** | 6 months*** | 9 months*** | 12 months |
|---|---|---|---|---|
| Vitamin A (μg/d) | 1084 ± 458 | 798 ± 314 | 1539 ± 528 | 881 ± 198 |
| Vitamin C (mg/d) | 385 ± 141 | 137 ± 48 | 310 ± 55 | 129 ± 70 |
| Vitamin B12 (mcg/d) | 4.5 ± 2.1 | 2.8 ± 0,3 | 4.5 ± 1.9 | 2.8 ± 0.8 |
| Zinc (mg/d) | 3.7 ± 0.4** | 4.7 ± 0.5** | 4.3 ± 0.9** | 4.1 ± 0.6** |
| Iron (mg/d) | 5.0 ± 1.3** | 5.5 ± 0.5** | 6.7 ± 0.7 | 5.6 ± 0.9** |
After the performance of restrictive and mixed surgical procedure such as RYGB, patients are normally instructed to adopt a liquid diet for periods of between 15 days and 2 months, depending upon the routines of each surgical group and upon eventual clinical manifestations.13–15 The standard recommendation in our institution is for a 30 day period of liquid diet, consisting of noncaloric coffee, tea, soft drinks, dietetic juices, and chicken broth.1,8. Afterwards, a full diet is introduced, so that by 60 days virtually all subjects are allowed to eat a complete and solid diet, which should provide most or all of their nutritional requirements. Just in case certain micronutrients are missing, a prophylactic multivitamin multimineral preparation (in the present series, Materna, Wyeth, São Paulo)16 is part of all follow-up protocols, including the current series.
It is true that this feeding style will permanently require a number of precautions, such as cutting all foodstuffs into tiny servings with slow and careful chewing, in order to avoid gastric obstruction and choking. High-lipid and energy-dense foods including highly caloric liquids are discouraged for rather obvious reasons, with emphasis on lean meats and other healthy protein sources, fruits, vegetables, and whole-grain bread and pasta.
Unfortunately quite a few subjects develop gastrointestinal derangements, most prominently vomiting, which may extend for longer than 1 year and involve from 8% to 49% of the operated population,15,17 such as here demonstrated. Food intolerances were not investigated in detail but are often detected as well. Meat and meat products, certain vegetables, fresh bread, and a few other foods may be rejected because they require prolonged mastication, and particularly because they seem to stick to the small gastric chamber and are thus associated with vomiting and choking episodes, which may frighten patients.13,17 Another source of valuable nutrients that is often discarded from the diet is milk (skimmed milk), which is easily swallowed but for unknown reasons becomes poorly tolerated after anti-obesity gastroplasty.13
All these pitfalls notwithstanding, the international experience with RYGB and other restrictive procedures suggests there are few reasons for concern, as adequate energy ingestion is typically achieved by 1 year or earlier. The results of Trostler et al,6 a landmark study, with ingestion of 694 ± 105 kcal/day after just 30 days were better than those in this study. Although the intake of patients in this study was not measured at 30 days, their intake of noncaloric fluids per protocol certainly amounted to a fraction of these calories.
By 3, 6, 9, and 12 months, Trostler et al 6 reported 535 ± 158, 1369 ± 262, 1597 ± 408, and 2008 ± 338 kcal/day, respectively, in contrast to the 529.4± 300.2, 710.9± 301.6, 833.2± 407.6 and 866.2± 342.7, respectively observed in this study. It can be noticed that values are similar only on the first trimester. From half a year onward, the discrepancy is in the order of 100%.
The findings of Naslund are much closer to those of the present investigation, with the 1-year mean intake not exceeding 1050 kcal.18 Similarly, an inadequate alimentation pattern after a purely restrictive intervention (vertical gastroplasty) was reported by Cooper et al2 and by Blake et al,19 notably within the first 6 months. Energy and protein intake were well below the USA Recommended Dietary Allowances20 during this period, and also risk for iron, zinc, folate and calcium deficiency was suggested.
It is worth emphasizing that hair loss, a harmless but esthetically troubling manifestation that affected a substantial proportion of the studied women, may be precipitated by reduced zinc intake,21 and several other nutrients probably underlie the manifestation of weakness and malaise (13% in the first 3 months).
Admittedly, dietary assessment in obese subjects is traditionally controversial, and the 24-h recall technique is not endorsed by all groups; some prefer more detailed instruments, such as the 72-h recall, or home forms filled in by the patients during each meal or snack. Nevertheless, there are reasons to accept the 24-h recall method as valid for postoperative follow-up, given the fact that patients are by that time less motivated to cheat or underestimate their intake.6,7,9,10
Other weaknesses in such assessments are the accuracy of computerized programs such as Virtual-Nutri (although calculations using standard tables were done in parallel) and interference of the multivitamin supplement. In the present study, we opted not to compute the contribution of the multivitamin supplement for several reasons: 1) many groups also omit such values;6,7,9,10 2) vomiting interferes with absorption, and the bioavailability of such medication after gastric bypass has never been determined; 3) compliance with the multivitamin protocol, despite all efforts, is far from ideal, as shown in a preliminary survey.22
Inappropriate dietary intake is amenable to prevention and treatment, and differences between various international experiences may be partially explained by efficacy of the multidisciplinary team in implementing and supervising correct postoperative alimentation.
In a public hospital dealing with a low-income and culturally unsophisticated population, as occurred in this study, some patients fail to return for follow-up, and many of those that come back do not strictly follow nutritional advice, which may have an adverse effect on general outcome.15 It is also likely that many subjects had an unbalanced diet preoperatively, and qualitative food prejudices are hardly changed after bariatric intervention, even with substantial guidance.6
Risk of postoperative undernutrition was thus demonstrated to be real in bariatric candidates within the described context up to at least 1 year, and spontaneous improvement in food intake was slow and inefficient. This situation could not be explained by unusual complications, as postoperative response to the intervention was within the expected range, with about 67% excess weight loss after 1 year. Similar results were observed with respect to gastrointestinal symptoms and drug requirements.5,6,8,10
Moreover, a parallel between insufficiently nourishing meals and poor quality of life during this period seems to exist, as indicated by the frequency of hair loss and systemic complaints in the population. Life-threatening nutritional derangements were not detected in this series but have been described in other reports.7,22
The reassessment of vitamin and mineral prescriptions is currently underway in our service, and new protocols are being written that aim to improve nutrition and health during the postoperative phase until successful dietary adaptation.
CONCLUSIONRisk of postoperative undernutrition was demonstrated up to 1 year following RYGB, and spontaneous improvement in food intake was slow and inefficient;
Specific protocols should be devised aiming to improve nutrition and health during the postoperative phase until successful dietary adaptation.