This study aimed to compare respiratory responses, focusing on the time-domain variability of ventilatory components during progressive cardiopulmonary exercise tests performed on cycle or arm ergometers.
METHODS:The cardiopulmonary exercise tests were conducted on twelve healthy volunteers on either a cycle ergometer or an arm ergometer following a ramp protocol. The time-domain variabilities (the standard deviations and root mean squares of the successive differences) of the minute ventilation, tidal volume and respiratory rate were calculated and normalized to the number of breaths.
RESULTS:There were no significant differences in the timing of breathing throughout the exercise when the cycle and arm ergometer measurements were compared. However, the arm exercise time-domain variabilities for the minute ventilation, tidal volume and respiratory rate were significantly greater than the equivalent values obtained during leg exercise.
CONCLUSION:Although the type of exercise does not influence the timing of breathing when dynamic arm and leg exercises are compared, it does influence time-domain ventilatory variability of young, healthy individuals. The mechanisms that influence ventilatory variability during exercise remain to be studied.
The maintenance of homeostasis is a continuous process dependent on multiple feedback mechanisms that operate around set points. Therefore, the variability of biophysical parameters is an integral aspect of physiological control. For example, fluctuations in the cardiac output and peripheral vascular resistance interact continuously, leading to the relative stability of the blood pressure values. Fluctuations in the cardiac output can be assessed via heart rate variability, which has been extensively studied and is related to the prognosis of cardiac diseases.1,2 The hemodynamic and respiratory components also fluctuate continuously. In this context, variability in the respiratory components could represent a relevant physiological phenomenon, although it is rarely addressed.3,4
In the last decade, heart-lung interactions have been identified as a key issue in heart failure.5 Several studies have suggested that the analysis of ventilatory parameters during exercise can add relevant information to the prognosis of heart failure patients.6–9 Despite the increasing number of recent publications discussing ventilatory abnormalities in heart disease, little is known about the physiological patterns and timing of breathing during exercise, even in healthy individuals. Neder et al10 published the only study that normalized the pattern and timing of breathing in healthy people of various ages and both genders during a progressive exercise test using a leg ergometer.
Classic respiratory parameters, such as ventilatory equivalents and the occurrence of periodic breathing, can confer relevant clinical and prognostic information in heart disease.4,9 The absence of a universally accepted definition of periodic breathing and the need to manually identify this phenomenon prevents the widespread use of periodic breathing as a prognostic criterion in heart failure.11 Clinical experience has shown that some heart failure patients exhibit ventilatory oscillations during exercise tests without achieving any previously established criteria for periodic breathing.4,12 Thus, it is conceivable that a time-domain analysis of variability during minute ventilation could be useful for detecting the light ventilatory oscillations that occur during exercise. However, the physiological pattern of ventilatory oscillations throughout exercise has not been previously described, despite the potential impact on the understanding of normal and abnormal physiology and its consequent clinical implications.
Although not routinely used, exercise testing can be very useful in clinical settings. However, most of the available ergometers depend on the function of the lower extremities. Assessing the physiological responses to upper-body exercise is the best option when evaluating both patients with lower extremity disabilities13 and high-performance kayaking, swimming and canoeing athletes.14,15 During dynamic upper-body exercise, movements of the arm and thorax may influence the pattern and timing of breathing. Therefore, the clinical status and the type of exercise performed could influence ventilation during exercise. Thus, the physiological responses to upper-body exercise testing must be described, such that tests can be correctly interpreted in clinical settings.
domain variability of the ventilatory variables (respiratory rate, minute ventilation and tidal volume) in healthy individuals during progressive cardiopulmonary exercise tests performed on cycle and arm ergometers.
METHODSVolunteersTwelve individuals from the population of hospital staff and university students were selected for this study. All of the selected individuals were considered healthy based upon a clinical evaluation (physical examination and clinical history) and a maximal exercise test performed on a cycle ergometer. None of the volunteers were engaged in regular physical exercise. The use of any medication (except oral contraceptives) and chronic diseases were considered to be the exclusion criteria for the study. None of the volunteers were accustomed to arm-crank exercise.
All of the volunteers gave written informed consent to participate in the study after a full explanation of the procedures and the potential risks. The investigation conformed to the principles outlined in the Declaration of Helsinki and was approved by the Institutional Research Ethics Committee on Human Research.
Study protocolThis study included three afternoon visits to our laboratory for each volunteer. Because the volunteers were previously accustomed to exercises performed on leg ergometers but not arm-crank exercises, each volunteer performed a maximal exercise test on an arm ergometer on the first visit. The first test was performed such that the subjects could become familiar with the laboratory and the facial mask that is used in the cardiopulmonary exercise tests.
On the second visit, the volunteers performed a maximal exercise test on a cycle ergometer (Excalibur Sport, LODE, Groningen, Netherlands), following an individualized ramp protocol. The initial workload selected was 50 W with increments of 15 seconds. The initial workload was set to achieve maximal effort in 8 to 12 minutes and considered the individual's level of physical activity, gender, body mass index and age. The individuals were instructed to maintain a pedaling frequency of 60±5 rotations per minute.
On the third visit, each individual performed a maximal cardiopulmonary exercise test on an electronically braked arm ergometer (Angio, LODE, Groningen, Netherlands) by following a linear increment ramp protocol with 2-W increments every six seconds (20 W/min), as previously validated.16 All of the tests started with a one-minute warm-up period with a workload of 30 W. Each individual was carefully positioned on the ergometer such that the rotational axis of the glenohumeral joint was at the same level as the axis of the ergometer's crank arm. The individuals were instructed to maintain a crank rate of 70 cycles/minute. The crank rate was used as the principal criterion for determining fatigue. Thus, failure to maintain a crank rate of more than 60 cycles/minute resulted in the termination of the test.
Cardiopulmonary exercise testThe cardiopulmonary exercise tests were performed with the gas exchange and ventilatory variables analyzed in a breath-by-breath manner using a calibrated, computer-based exercise system (Ultima CardiO2 System, Medical Graphics Corporation, Minnesota, USA). The O2 and CO2 analyzers were calibrated before each test using a reference gas (12% O2; 5% CO2; nitrogen balance). The pneumotachograph was also calibrated with a 3-L syringe using various flow profiles.
During each cardiopulmonary exercise test, a 12-lead electrocardiogram was continuously recorded (Cardioperfect, Welch Allin, USA), and the heart rate was registered.
The oxygen consumption (VO2), carbon dioxide production (CO2), tidal volume (Vt), inspiratory time (Ti), expiratory time (Te), total respiratory time (Ttot), duty cycle (Ti/Ttot), mean respiratory flow (Vt/Ti) and respiratory rate (RR) were registered in a breath-by-breath manner. The derived variables [minute ventilation (Ve), respiratory equivalents for oxygen (Ve/VO2) and carbon dioxide (Ve/VCO2)] were calculated online (Breeze Software 6.4.1, Medical Graphics, USA). RR, Ve, Ttot, Ti, Te, Ti/Ttot and Vt/Ti were analyzed at 40%, 60%, 80% and 100% of the maximal ventilatory intensity for each individual.
The time-domain variabilities of Ve, RR and Vt during exercise were calculated as the standard deviation (SD) and root mean square successive difference (RMSSD) of each variable. Both calculations were normalized by the number of breaths (SD/n and RMSSD/n, respectively) because the duration of the test could have influenced these results.17
Statistical AnalysisStatistical analysis was performed using the software Statistica 7.0 (Statsoft Inc., Oklahoma, USA). The descriptive data are presented as the mean ± standard error of the mean. The variables from the cardiopulmonary exercise tests were normally distributed when analyzed by the Shapiro-Wilk W test. The variables were obtained during the leg or arm exercises at various intervals (40%, 60%, 80% and 100% of maximal ventilation). The data were compared using two-way ANOVA followed by the post hoc Bonferroni test. The time-domain ventilatory variability variables during the exercise tests with both ergometers were compared using paired Student's t-tests. Significance was set at p<0.05.
RESULTSTwelve healthy individuals completed the study (6 males; age, 27±1 years; body mass index, 22.7±0.7 kg/m2). All of the tests were maximal, with a respiratory quotient greater than 1.1 (Table 1). The peak exercise variables from both tests are shown in Table 1. Although there was no difference between the peak heart and respiratory rates when both tests were compared, the peak VO2 was greater when the leg exercise was performed, as expected. The analysis of the ventilatory variables normalized to the peak power (Table 1) clearly shows that the same load elicits more ventilatory responses during arm exercise than during leg exercise.
Peak exercise data during graded maximal cardiopulmonary leg or arm exercise tests (n = 12).
| Arm exercise | Leg exercise | p-value | |||||
|---|---|---|---|---|---|---|---|
| VO2 (mL/kg/min) | 26.0 | ± | 1.8 | 37.8 | ± | 1.8 | <0.001 |
| VCO2 (mL/kg/min) | 34.0 | ± | 2.5 | 46.2 | ± | 2.2 | <0.001 |
| Ve (L/min) | 78.9 | ± | 9.3 | 99.9 | ± | 32.1 | <0.001 |
| Respiratory rate (breaths/min) | 52 | ± | 4 | 52 | ± | 2 | 0.80 |
| Vt (L) | 1.53 | ± | 0.18 | 1.95 | ± | 0.16 | <0.01 |
| Heart rate (beats/min) | 167 | ± | 4 | 177 | ± | 5 | 0.13 |
| Power (W) | 116 | ± | 13 | 225 | ± | 20 | <0.001 |
| Respiratory quotient | 1.37 | ± | 0.03 | 1.26 | ± | 0.03 | 0.20 |
| Ve/power (L/min/W) | 0.69 | ± | 0.05 | 0.45 | ± | 0.02 | <0.001 |
| Respiratory rate/power(breaths/min/W) | 0.50 | ± | 0.06 | 0.25 | ± | 0.02 | <0.001 |
| Vt/power (mL/W) | 13.27 | ± | 0.74 | 8.77 | ± | 0.32 | <0.001 |
VO2: oxygen consumption; VCO2: carbon dioxide production; Ve: minute-ventilation; Vt: tidal volume.
p-value refers to the result of the paired Student's t-test.
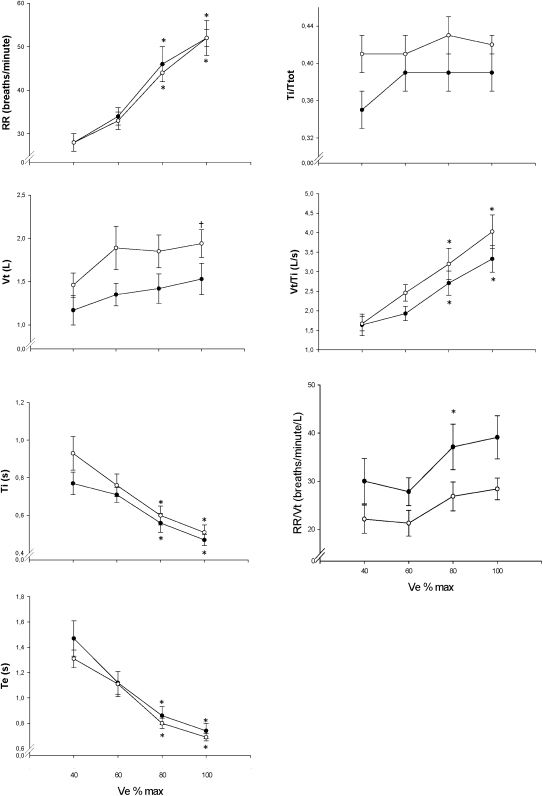
Figure 1 shows there was no difference in the timing of breathing throughout the exercise when both ergometers were compared (p>0.05), but the analysis in Table 2 shows greater time-domain variability in the ventilatory parameters during arm-crank exercise than during leg exercise.
The timing of breathing during incremental exercise performed with the legs (white circles) or arms (black circles), expressed as a function of the relative ventilatory responses of healthy volunteers (n = 12). The vertical bars represent standard errors. * p<0.01 vs. 40% with the same ergometer; †p<0.01 vs. the arm ergometer. VT: tidal volume; RR: respiratory rate; Ti: inspiratory time; Te: expiratory time; Ttot: total respiratory time.
The time-domain ventilatory variability of healthy individuals (n = 12) during graded maximal cardiopulmonary arm or leg exercise tests.
| Variable (*103) | Arm exercise | Leg exercise | p-value | ||||
|---|---|---|---|---|---|---|---|
| Respiratory rate | |||||||
| SD/n (breath2/min) | 72.2 | ± | 10.7 | 42.8 | ± | 3.5 | 0.021 |
| RMSSD/n (breath2/min) | 90.9 | ± | 14.3 | 38.3 | ± | 3.5 | 0.001 |
| Tidal volume | |||||||
| SD/n (l/min/breath) | 2.6 | ± | 0.2 | 1.5 | ± | 0.2 | <0.001 |
| RMSSD/n (l/min/breath) | 3.4 | ± | 0.3 | 1.8 | ± | 0.2 | <0.001 |
| Minute ventilation | |||||||
| SD/n (l/breath) | 124.6 | ± | 2.6 | 93.7 | ± | 2.0 | 0.002 |
| RMSSD/n (l/breath) | 112.9 | ± | 2.8 | 59.5 | ± | 1.4 | <0.001 |
SD: standard deviation; RMSSD: root mean square successive difference; n: the number of breaths during the exercise test.
p-value refers to the result of the paired Student's t-test.
Neder et al10 have previously described the timing of breathing in healthy volunteers ranging from 20 to 80 years old. Although this normative study10 provided us with clinically relevant reference values that enabled the comparison and evaluation of the timing of breathing during exercise tests performed on the cycle ergometer, there was still a lack of knowledge regarding whether similar results would be found in other types of exercise. The analysis of the results of this study shows that the type of exercise (dynamic leg or arm exercise) does not influence the timing of breathing during maximal progressive exercise tests, even during maximal ventilation. Cerny & Ucer18 found that heavy arm exercise elicits a greater respiratory rate than leg exercise. We believe that their results18 concerning the respiratory rates and inspiratory and expiratory times during heavy exercise differ from ours because “heavy exercise” is defined differently between the studies. Our study compared arm and leg exercise in the same fraction of maximal minute ventilation. In their study, absolute minute ventilation values were used in the comparison. Thus, in their study,18 when the volunteers were pedaling at the same maximal minute ventilation achieved during arm cranking, their effort was not as intense as the effort described in our study.
Given that the peak power output is much greater during leg exercise than during arm exercise, it is necessary to evaluate the variables and normalize them to the peak power output. Arm exercise elicits greater minute ventilation, respiratory rates and tidal volumes than leg exercise at the same level of effort, as previously shown by Sawka et al.19
The cardiopulmonary exercise test is increasingly being used as a diagnostic and prognostic tool in clinical practice. Recently, more attention has been paid to the exercise ventilatory responses of patients with heart disease. A recent study9 emphasized the role of the ventilatory equivalent of the carbon dioxide output in determining prognosis of heart failure patients. After evaluating the various classical prognostic predictors, the same study9 concluded that the most powerful predictive model of early mortality and morbidity in heart failure is the combination of the lowest elevated Ve/VCO2 and the presence of periodic breathing during exercise tests. Nevertheless, the absence of a universally accepted definition of periodic breathing and the need to manually identify this phenomenon impairs the widespread use of periodic breathing as a prognostic criterion for heart failure.11 It is unknown whether a graded or quantitative assessment of periodic breathing would be able to refine the prognostic information that is usually produced by the presence or absence of this phenomenon. We decided to apply mathematical methods that are traditionally used to evaluate time-domain heart rate variability to analyze the ventilatory variability during exercise because periodic breathing is diagnosed by criteria that, together, depict the variability of ventilation throughout a graded exercise test. We have previously used the same methods to study time-domain ventilatory variability in cardiac disease.17 We found that breath-by-breath minute ventilation and respiratory rate variability during exercise are inversely correlated to the left ventricular ejection fraction in heart failure. Hence, patients with lower ejection fractions exhibit more ventilatory variability during a graded symptom-limited exercise test. In this study, we applied the same method and found that the ventilatory variability of healthy individuals is greater during arm exercise than during leg exercise. To our knowledge, this is the first study to evaluate ventilatory variability during arm exercise but was not designed to determine the mechanisms of this phenomenon. It is important to note that all of the volunteers evaluated in this study were healthy. This is the first study to describe the physiological ventilatory variability responses in healthy individuals during upper- and lower-body exercises. Although this study does not have the characteristics of a normative study, its results can be used as reference values when ventilatory variability is evaluated in other populations.
The mechanisms involved in Cheyne-Stokes respiration and periodic breathing, such as hypocapnia, increased central and peripheral chemosensitivity20 and pulmonary blood flow fluctuations21 (key mechanisms in heart failure), are probably not useful for understanding physiological ventilatory variability during exercise in healthy subjects. It remains to be discovered why the respiratory variables are unstable during exercise and why there are differences in the time-domain ventilatory variabilities of leg and arm exercise. The responses of the respiratory system to physical exercise represent one of the main challenges in the study of homeostasis.22
Arm exercise elicits more lactate accumulation than leg exercise at the same power output,23 a greater rate of perceived effort and more stimulation to breathe from force-sensing mechanoreceptors in the joints or from greater sympathetic stimulation.23,24 Such factors are usually accepted as the reasons for the elevated sensations of breathlessness during arm work.18 The increased perturbation in the autonomic system, the blood pH and the effort perception elicited by arm exercise when compared to leg exercise at the same output could lead to an increasingly difficult equilibrium in the respiratory responses, which might explain the greater ventilatory variability during progressive arm exercise.
Arm exercise elicits more inputs from the afferent muscle fibers than leg exercise, leading to different autonomic responses when both types of exercise are compared. This difference is independent of the active muscle mass but relies on the number and/or sensitivity of the afferent receptors in the upper body.
Autonomic modulation could be a potential mechanism that influences ventilatory variability during exercise. A phenomenon called cardioventilatory coupling has been proposed. This describes a condition in which heartbeats entrain the respiratory rhythm, triggering inspiratory onset by unknown afferent cardiovascular pathways.25,26 Thus, changes in autonomic cardiovascular modulation and heart rate variability could potentially influence the respiratory rhythm and ventilator variability. Few studies have compared autonomic modulation during arm vs. leg exercise. There is more sympathetic nervous activity during maximal27 or submaximal28 lower-body exercise than during upper-body exercise.
Leicht et al29 demonstrated that, during moderate steady-state exercise, the heart rate variability was reduced from its resting levels but with more heart rate variability during upper-body exercise than during lower-body exercise. The increased heart rate variability during arm exercise might reflect more respiratory sinus arrhythmia.29 In contrast, Tulppo et al27 found that dynamic arm exercise results in more a rapid withdrawal of the vagal outflow than dynamic leg exercise. We have studied the time-domain variability of ventilation during a graded maximal exercise test, from rest to peak exercise. Hence, our analysis included the moments during the exercise test in which the vagal activity was minimal. Nevertheless, the increased vagal modulation during arm exercise between rest and moderate effort may, at minimum, have the influenced ventilatory variability throughout the test. Although evaluating autonomic modulation during exercise was beyond the scope of this study, it is a potential mechanism and remains to be studied.
LimitationsSome operational and technical aspects could have influenced the results obtained in this study. The subjects were not subjected to rest pulmonary function tests before entering the study. Given that none of the subjects had any history of pulmonary disease or smoking and that individual tests were used in the comparisons, the absence of rest pulmonary function tests does not seem to be a major issue that influenced the results. All of the breath-by-breath data were collected with a face mask. Thus, the use of the face mask cannot explain the different results when the arm and legs exercise were compared. The use of a mouthpiece and nose clip is known to influence the depth and rate of breathing.30 Although this effect appears to be restricted to the lower levels of exercise,31 it seems reasonable not to interchangeably compare the ventilatory variability results recorded using the mask, mouthpiece and canopy.
Trained volunteers sometimes tend to match their breathing pattern to the cycling rate.32 The pedaling and arm-crank rotation rates were fixed at values of more than 60 cycles per minute. Given that the respiratory rates did not reach such high frequencies, it is clear that the cycling rate could not have influenced the ventilatory variability in this study.
Finally, each individual performed only one maximal exercise test with each ergometer. Thus, we were unable to evaluate the inter-test reproducibility of the respiratory responses during exercise. Given that the methods used to quantify the ventilatory variability (RMSSD and SDNN) are purely mathematical calculations and are not evaluator-dependent, the intra-test reproducibility would be meaningless and, thus, was not measured.
CONCLUSIONSThe timing of breathing was not influenced by the type of exercise performed when dynamic arm and leg exercises were compared. The time-domain ventilatory variabilities of young, healthy, sedentary individuals was greater during the maximal graded exercise test performed on the arm ergometer when compared to the leg ergometer. The mechanisms that influence ventilatory variability during exercise remain to be studied.