We aimed to evaluate whether the inclusion of videothoracoscopy in a pleural empyema treatment algorithm would change the clinical outcome of such patients.
METHODS:This study performed quality-improvement research. We conducted a retrospective review of patients who underwent pleural decortication for pleural empyema at our institution from 2002 to 2008. With the old algorithm (January 2002 to September 2005), open decortication was the procedure of choice, and videothoracoscopy was only performed in certain sporadic mid-stage cases. With the new algorithm (October 2005 to December 2008), videothoracoscopy became the first-line treatment option, whereas open decortication was only performed in patients with a thick pleural peel (>2 cm) observed by chest scan. The patients were divided into an old algorithm (n = 93) and new algorithm (n = 113) group and compared. The main outcome variables assessed included treatment failure (pleural space reintervention or death up to 60 days after medical discharge) and the occurrence of complications.
RESULTS:Videothoracoscopy and open decortication were performed in 13 and 80 patients from the old algorithm group and in 81 and 32 patients from the new algorithm group, respectively (p<0.01). The patients in the new algorithm group were older (41±1 vs. 46.3±16.7 years, p = 0.014) and had higher Charlson Comorbidity Index scores [0(0-3) vs. 2(0-4), p = 0.032]. The occurrence of treatment failure was similar in both groups (19.35% vs. 24.77%, p = 0.35), although the complication rate was lower in the new algorithm group (48.3% vs. 33.6%, p = 0.04).
CONCLUSIONS:The wider use of videothoracoscopy in pleural empyema treatment was associated with fewer complications and unaltered rates of mortality and reoperation even though more severely ill patients were subjected to videothoracoscopic surgery.
Pleural empyema (PE) is a significant clinical condition with a mortality rate approaching 20% (1). PE treatment consists of early drainage of the pleural space and the use of antibiotics (2). Although open thoracotomy is the classical approach for PE treatment, tube thoracostomy is generally indicated for patients with early-stage empyema, whereas patients with pleural loculations or a trapped lung should ideally undergo pleural decortication (1,2). More recently, however, video-assisted thoracic surgery (VATS) has become an attractive alternative, as many authors claim that this technique serves as a minimally invasive procedure to provide reduced morbidity and postoperative pain, improved cosmetics and fewer complications (3,4).
Previous case series have compared the use of VATS with that of traditional open decortication (OD) head-to-head 5–15)for PE treatment. These comparative studies demonstrated the usefulness of VATS and its advantages related to shorter postoperative hospital and intensive care unit (ICU) stays (5,7,8), less intense postoperative pain (9,11,12) and reduced morbidity (6,8). Such studies have also suggested that videothoracoscopy should replace open decortication in most situations and that it should be emphasized in the PE management algorithm. Nonetheless, the question as to whether the wider use of VATS would improve clinical outcomes in daily practice remains unanswered. Therefore, the purpose of the present study was to evaluate whether the implementation of VATS as part of a PE treatment algorithm would alter the clinical outcomes of such patients in routine clinical care.
MATERIALS AND METHODSThis study implemented quality-improvement research and conducted a retrospective study that compared the effects of PE treatments performed during two different periods of time. During the first period (January 2002 - September 2005), we used an algorithm to treat PE in which patients preferably underwent OD. Then, in 2005, our division acquired a dedicated video system, and we changed our protocol. During this second period (October 2005 - December 2008), a new algorithm was used that included VATS as the procedure of choice.
We sought to include all consecutive patients who underwent PE surgical treatment from January 2002 to December 2008 at the Division of Thoracic Surgery of Clinics Hospital at the University of São Paulo Medical School. We reviewed the records of our division using the terms pleural empyema, parapneumonic effusion, pleural decortication, thoracotomy and video-assisted thoracic surgery and retrieved a total of 234 patients. The charts of these patients were recovered. The exclusion criteria included a complete absence of data in a patient's charts and equivocal inclusion in the records of our division (misclassification). Patients who underwent open thoracostomy but not VATS or OD were not included in this study. The study protocol was reviewed and approved by the local institutional ethics committee (CAPPesq number 0422/10).
Treatment algorithmsThe management of patients with suspected pleural infection or disease at our institution was performed in accordance with the Brazilian Guidelines for Pleural Empyema Management (16), which are similar to the British Thoracic Society guidelines for the management of pleural infection (17). Briefly, patients with a pleural effusion demonstrating purulent fluid or aglucose level <40 mg/dL, lactate dehydrogenase level>1,000 IU/L, or positive Gram stain or culture were selected to undergo thoracostomy tube drainage and an intravenous antibiotics regimen. In addition, patients whose chest computed tomography (CT) scans or ultrasonography results suggested loculated pleural effusion, as well as those whose previous treatment with a chest tube had failed, were selected to receive pleural decortication. This treatment algorithm was strictly adhered to by the surgeons of our department, such that patient selection for operative procedures was standardized and homogeneous throughout the study period.
This study involved a comparison between two time periods, during which different algorithms were used to treat PE. During the first period, which ranged from January 2002 to September 2005, the algorithm (termed old algorithm) considered OD to be the procedure of choice for pleural decortication due to PE. Patients exhibiting significant medical comorbidities that resultedin a prohibitively highoperative risk received open thoracostomy (18) and were not included in this study. During this first period, VATS was only performed in a few sporadic, randomly selected mid-stage cases, as the surgical team was constrained by video system availability.
In 2005, our division acquired an exclusive video system, which provided the opportunity to fully incorporate VATS as part of the PE treatment algorithm. In this second period, ranging from October 2005 to December 2008, the algorithm (termed new algorithm) considered VATS to be the procedure of choice, and our team resorted to OD only for patients with a very thick pleural peel (>2 cm), as observed by CT scan. The indications for open thoracostomy remained the same as those during the first period of the study.
Surgical techniquesThe entire patient series was operated on under general anesthesia, using a double-lumen tube for selective ventilation. For the patients undergoing OD, the thorax was approached via standard lateral thoracotomy. After debridement was accomplished, one or two chest tubes (28F) were inserted into the lower part of the cavity through separate incisions. For patients receiving the VATS approach, we routinely administered a 2-cm high incision (fourth or fifth intercostal space) to enable proper visual cavity exploration. A 10-mm trocar was inserted following digital exploration. By direct visualization, two more incisions were made in the fifth and eighth intercostal spaces. The videothoracoscope and the trocar were then moved to the lower incision. Additionally, as much of the visceral and parietal pleural peel was removed as possible to perform decortication. The procedure was converted to OD if adequate decortication was not achieved or if complications occurred that could not be approached by VATS. After the lung was sufficiently reexpanded and examined for air leakage, one or two chest tubes (28F) were placed anteriorly or posteriorly under direct videothoracoscopic vision.
The patients were sent either to the ICU or to the ward, depending on their degree of illness and performance status; however, the OD or VATS approach itself was not considered to be an ICU indication. Antibiotic therapy continued postoperatively, as guided by the microbiologic analysis of intraoperative specimens. Pain management (19) and respiratory therapy (20) were standardized and delivered in a homogeneous fashion throughout the study. Chest radiographs were obtained at the first postoperative day, following chest tube removal and subsequently depending on the clinical course of each patient prior to medical discharge. The chest tubes were removed only after the cessation of air leakage or daily drainage of less than 200 ml of serous fluid. Prolonged air leakage was dealt with by attaching a Heimlich valve, whichenabled outpatient follow-up.
As previously mentioned, VATS became the procedure of choice for PE treatment during the seven-year study period. Throughout this period, minor changes were also made regarding antibiotic therapy and intensive care. However, the pain management and respiratory therapy protocols did not change significantly.
Data collectionPatient data were retrospectively collected from the patient charts for the current study, and the following patient information was obtained: age; gender; length of preoperative hospital stay (time spent between the first day of hospitalization and the surgical procedure); preoperative thoracostomy tube drainage; type of surgical treatment (VATS or OD); PE etiology; time spent in surgery (including in-room anesthesia time, surgical procedure time and in-room anesthesia recovery time); length of postoperative hospital stay; postoperative chest tube time (defined as when the chest tube was removed); length of postoperative ICU stay; and the occurrence of complications, reinterventions in the pleural space and mortality (in-hospital deaths and those within 60 days of medical discharge).
OutcomesThe two main outcomes evaluated were failure of the surgical therapy and the occurrence of complications. Treatment failure was defined as the occurrence of reintervention in the pleural space (i.e., reinsertion of the chest tube, open thoracostomy or redecortication) or death (in-hospital deaths and those within two months of hospital discharge). The incidence of complications was thoroughly investigated using the available patient charts, particularly those previously identified as demonstrating intraoperative bleeding (requiring blood transfusion), deep vein thrombosis, pulmonary embolism, prolonged air leakage (>sevendays), cardiac arrhythmia, subcutaneous emphysema, surgical wound infection or seroma and bronchopneumonia (defined as a lung infection that developed into non-pneumonic PE or moved to the contralateral lung).
The following variables were also analyzed to assess the overall effectiveness of the strategies employed during both time periods: postoperative hospital stay, thoracostomy tube drainage time and ICU stay.
Data analysisThe studied patients were divided into the following two groups, according to the algorithm used to define their surgical procedure: the OA (old algorithm) and NA (new algorithm) groups.
Statistical significance was set at 0.05. For qualitative variables, Fisher's exact test or the Chi-square test was used as appropriate. For quantitative variables, the Mann-Whitney U test was used for non-parametric variables, whereas the non-paired Student's t-test was used for parametric variables. Potential outcome predictive factors were evaluated using a univariate analysis. Those factors with p-values<0.1 were included in the multivariate analysis to compose a logistic regression model. All of the tests were performed using the Stata® Inc. software, version 11, 2009.
RESULTSA total of 234 patients were identified from the records of the thoracic surgery division at our institution, but only 206 of these cases were selected for the current study. The flow chart shown in Figure 1 depicts the reasons for patient exclusion at all stages of the study, the methods used for group division and the main outcome results.
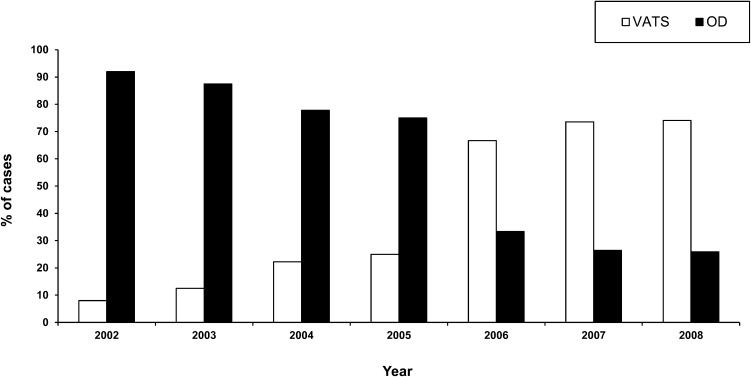
The characteristics of the patients included in the study are presented in Table 1. Parapneumonic PE was the most frequent type of PE etiology (n = 131), and community-acquired pneumonia accounted for 72.5% (n = 95) of such cases. Similar rates were found in both patient groups (OA Group: 42 patients, 73.6% vs. NA group: 53 patients, 71.6%). The patients in the NA group were older and had significantly higher ASA-PS (American Society of Anesthesiologists Physical Status) (21) and Charlson Comorbidity Index (CCI) (22) scores than the patients in the OA group. As expected, OD was more common in the OA group, whereas the use of VATS predominated in the NA group. The distribution of these procedures within the examined time period is shown in Figure 2.
Total sample and group characteristics.
| Variable | Total | OA Group | NA Group | p-value |
|---|---|---|---|---|
| No. patients | 206 | 93 | 113 | |
| Gender (M/F) | 150/56 | 67/26 | 83/30 | 0.94 |
| Age (years) | 44.1±16.8 | 41±17 | 46.3±16.7 | 0.014 |
| Etiology (n) | ||||
| Parapneumonic | 131 (63.5%) | 57 (61.2%) | 74 (65.4%) | |
| Post-Thoracic Procedures* | 24 (11.6%) | 9 (9.6%) | 15 (13.2%) | |
| Tuberculous | 24 (11.6%) | 12 (12.9%) | 12 (10.6%) | |
| Infected Hemothorax+ | 20 (9.7%) | 12 (12.9%) | 8 (7.1%) | |
| Subphrenic abscess† | 5 (2.42%) | 2 (2.1%) | 3 (2.6%) | |
| Unknown | 2 (0.9%) | 1 (0.1%) | 1 (0.8%) | |
| Hemithorax (R/L/Bilat) | 120/82/4 | 51/40/2 | 69/42/2 | 0.66 |
| Preoperative hospital stay (days) | 14.8±19.2 | 15.1±17.9 | 14.6±20.4 | 0.85 |
| Previous Chest Tube | 92 (44.6%) | 44 (47.3%) | 48 (42.4%) | 0.24 |
| Preoperative drainage time (days) | 12.8±10.3 | 11±7.3 | 14±11.9 | 0.28 |
| ASA-PS (p25-p75) | 2 (2-3) | 2 (1-3) | 2 (2-3) | 0.013 |
| CCI (p25-p75) | 1 (0-4) | 0 (0-3) | 2 (0-4) | 0.032 |
| OD/VATS | 112/94 | 80/13 | 32/81 | <0.001 |
The data are presented as either n(%), median ± standard deviation or p50 (p25-p75). OA, old algorithm; NA, new algorithm; M, male; F, female; R, right; L, left; Bilat, bilateral; ASA-PS, American Society of Anesthesiologists Physical Status; CCI, Charlson Comorbidity Index; OD, open decortication; VATS, Video-Assisted Thoracic Surgery; *, thoracic procedures included: pulmonary lobectomy (n = 7), pleurodesis (n = 5) and others (n = 9);+, causes included thoracic trauma (n = 15) and others (n = 5); †, causes included hepatic abscess (n = 4) and pancreatic-pleural fistula (n = 1).
Use of video-assisted thoracoscopic surgery (VATS) and open decortication (OD) for cases of pleural empyema according to year of the study. Each bar represents the relative percentage of cases performed using the VATS approach (white bar) or the open approach (black bar). It is noteworthy that the number of VATS cases performed tended to increase over time, whereas those using OD demonstrated a steady decline.
Pleural liquid bacterioscopic results were obtained in 94.67% (n = 192) of the patients, and these results tested positive in 30.21% of such cases (n = 58). These results revealed Gram-positive bacteria in 26.04% (n = 50) of the cases and Gram-negative bacteria in 8.85% (n = 17). Pleural liquid culture results were obtained in 95.15% of patients (n = 196), and these results tested positive in 44.38% of such cases (n = 87). The most frequently identified bacterium was Staphylococcus aureus (17.85%, n = 35). Anaerobic microorganisms were identified in 6.63% (n = 13) of the patients, and Streptococcus pneumoniae was identified in 0.51% of the cases (n = 1). No significant difference was observed between the groups regarding these microbiological results.
The variables considered for the determination of treatment efficacy are presented in Table 2. The treatment failure rates were similar in both groups, and no significant results were found when the groups were adjusted for age and the presence of comorbidities. All of the deaths occurred in-hospital, as none occurred after patient discharge. The causes for patient death and pleural space reintervention procedures are listed in Table 3. A higher complication rate was observed among the patients in the OA group. Even after excluding intraoperative blood transfusion as a complication, this rate remained significantly higher among the patients in the OA group (24.73% vs. 14.15%, p = 0.02). The patients in the NA group experienced longer postoperative ICU stays (p = 0.045) and tended to have greater postoperative thoracostomy tube drainage times (p = 0.09). Overall, the OD conversion rate was 2.91% (n = 6), as observed in the NA group. The causes for conversion included inadequate lung reexpansion that required further decortication in five cases and accidental diaphragmatic injury in one patient. In addition, one patient experienced an intraoperative diaphragmatic injury that was able to be fixed without conversion.
Variables defining treatment efficacy.
| Variable | Total(n = 206) | OA Group (n = 93) | NA Group (n = 113) | p-value |
|---|---|---|---|---|
| Treatment Failure | 46 (22.3%) | 18 (19.3%) | 28 (24.7%) | 0.35 |
| Patient Death | 31 (15.1%) | 13 (13.9%) | 18 (15.9%) | |
| Pleural Space Reintervention | 19 (9.2%) | 6 (6.45%) | 13 (11.5%) | |
| Cases with complications* | 83 (40.2%) | 45 (48.3%) | 38 (33.6%) | 0.04 |
| Total number of complications | 110 | 60 | 50 | |
| Intraoperative blood transfusion | 63 | 34 | 29 | |
| DVT | 4 | 1 | 3 | |
| Prolonged air leak | 13 | 6 | 7 | |
| Chylothorax | 1 | 0 | 1 | |
| Diaphragmatic lesion | 2 | 0 | 2 | |
| High Response Atrial Fibrillation | 1 | 1 | 0 | |
| Bronchopneumonia | 11 | 10 | 1 | |
| Surgical Wound Infection | 7 | 3 | 4 | |
| Seroma | 2 | 2 | 0 | |
| Subcutaneous Emphysema | 6 | 3 | 3 | |
| Operation Room time (min)+ | 200.8±87 | 198.6±82.6 | 202.7±90.8 | 0.74 |
| ICU postoperative stay (days) | 1 (0-2) | 1 (0-2) | 1 (0-3) | 0.045 |
| Thoracostomy tube drainage (days) | 6 (5-11) | 6 (5-8) | 7 (5-14) | 0.09 |
| Postoperative hospital stay (days) | 9 (7-16) | 8 (7-14) | 10 (7-19) | 0.14 |
The data are presented as either n (%), median ± standard deviation or p50 (p25-p75). OA, old algorithm; NA, new algorithm; ICU, intensive care unit; DVT, deep vein thrombosis; *, number of patients with at least one complication; ?time spent between the patient's entrance to and exit from the surgical room.
Causes of death and new pleural procedures performed as reinterventions.
| Variable | Total(n = 206) | OA Group (n = 93) | NA Group (n = 113) | p-value |
|---|---|---|---|---|
| Patient Death | 31 (15.1%) | 13 (13.9%) | 18 (15.9%) | 0.69 |
| Multiple Organ Failure | 25 | 12 | 13 | |
| Respiratory Insufficiency | 2 | 1 | 1 | |
| Non-pulmonary cause | 4 | 0 | 4 | |
| Postoperative Day Death | 13 (8.5-29) | 11 (9-14) | 18.5 (8.5-33.5) | |
| Pleural Space Reintervention | 19 (9.2%) | 6 (6.4%) | 13 (11.5%) | 0.21 |
| Chest tube | 6 | 1 | 5 | |
| Open Thoracostomy | 6 | 4 | 2 | |
| Decortication | ||||
| VATS | 2 | 0 | 2 | |
| OD | 5 | 1 | 4 | |
| Postoperative Day Reintervention | 23 (18.2-36.7) | 30 (21.5-54.2) | 22.5 (18-33.2) |
The data are presented as either n (%) or p50 (25-75). OA, old algorithm; NA, new algorithm; VATS, Video-Assisted Thoracic Surgery; OD, open decortication.
After performing aunivariate and logistic regression analysis including all of the significant predictors, only CCI was shown to correlate with both treatment failure (Odds Ratio = 1.26, 95% Confidence Interval: 1.08-1.47, p = 0.003) and death (Odds Ratio = 1.202, 95% Confidence Interval: 1.04-1.37, p = 0.008). In addition, chest tube insertion prior to the surgical procedure correlated with pleural space reintervention performance (Odds Ratio = 2.96, p = 0.03).
DISCUSSIONIn this study, the implementation of VATS as the procedure of choice in the PE management algorithm did not change the overall outcome of patients in a tertiary care facility, and the mortality and reintervention rates were similar for both algorithms. Nonetheless, during the second time period, the complication rate decreased significantly even though the profile of the patients undergoing pleural decortication had changed, as older and more debilitated patients (according to the ASA and Charlson Comorbidity Index values) were operated on during this time. These patient differences maybe explained by the fact that the clinicians felt more confident sending severely impaired patients to receive a minimally invasive procedure than an open surgery.
Several of the results obtained in this study were outside of the ranges commonly reported in previous case series. First, the mortality rate of 15.04% identified in the current study was higher than the typical range from 0 to 3.32 % (5–7,9,11). We ascribe this finding to the following factors: a) the characteristics of our population, as there were a greater number of comorbidities and cases proceeding from the ICU; b) the inclusion of patients with PE of an etiology other than parapneumonic, notably post-thoracic procedures (11.6%) and tuberculosis (11.6%) empyemas; c) the presence of patients with malignant pleural effusions, for whom it is significantly more difficult to achieve full lung expansion (23,24); and d) the definition of mortality as in-hospital death or with 60 days after medical discharge, compared with perioperative death, which has been commonly used in other case series (5,6,8,9,11,12,14). For the MIST-1 trial, Maskell et al. used similar outcome definitions and reported similar empyema-related mortality rates (14.5% at three months) (25). Second, the overall rate of cases presenting at least one complication was 40.2%, which was higher than the typical range reported in the literature (11 to 25%) (10–12). Again, this discrepancy was likely related to the specific definitions used. Similarly to previous studies (13), we considered intraoperative blood transfusion to be a complication, as it is often a surrogate for intraoperative bleeding. Furthermore, blood transfusions correlate with procedure morbidity, involve potential intrinsic complications and increase the financial cost of the surgical procedure. Ultimately, blood transfusions accounted for 57.27% of all of the complications identified, whereas the most frequent complication in previous series was prolonged air leakage (11). However, as shown previously in the literature (6,8), the implementation of VATS led to fewer complications; even after excluding blood transfusion as a potential complication, the difference between the groups remained significant, and the overall complication rate was 18.93%. Finally, the microbiological findings of the current study were particularly intriguing. Not only was the pleural fluid culture positivity lower than those reported in other series (44.38% vs. 53.95 - 76.1%) (8,25) but the culture profiles were also quite unusual. For example, S. pneumoniae was identified in only one case, although it has been commonly referred as an important etiological agent [present in 8.37 - 14.95% of cases] (8,25), and S. aureus was the most frequent bacterial strain isolated in our series. These facts may reflect the large number of ICU patients in our study and the effects of early antibiotic use.
Previous studies have extensively described the advantages of VATS over OD, which include shorter postoperative hospital stays (5,7,8), a more rapid return to work (11), reduced thoracostomy tube drainage time (6), decreased surgical time (6,7,9), less perioperative pain (9,11,12), a reduced complication rate (6,8), decreased postoperative air leakage (11,13) and reduced mortality (8,13). Therefore, we had hypothesized that there would have been improvements in the measured outcomes of PE treatment after the implementation of VATS as a standard procedure, but only the complication rates were shown to improve. Apart from the outcome definition issue, the following reasons may serve to explain our findings. First, our study represented a standard clinical practice scenario (outcomes assessment study), in which the results were influenced by several unknown confounders. Second, during the second period, more severely affected patients were operated on, which may have masked the advantages of the VATS method.
Among our results, one unexpected finding was the longer postoperative ICU stays during the second period. Older age and a greater number of comorbidities may have lengthened the time spent in the ICU for these patients even though they had fewer postoperative complications than the patients in the OA group. Another surprising finding was the reintervention rate associated with the NA group, which, although not significant, was substantially higher than that observed in the OA group. However, the reintervention rate of the NA group rate may have been overestimated because, at the beginning of the wider use of VATS, the surgeons were not as confident with this method and were more prone to perform additional procedures in such patients. In support of this assumption is the fact that, during the second period, chest tube insertion (a minor procedure) was the most frequent pleural reintervention, whereas open thoracostomy was the most frequent type of reintervention during the first period. In our division, open thoracostomy is considered to represent the last resort for empyema management. Alternatively, the greater reintervention rate during the second period may have been associated with incomplete decortications, which could also explain the longer chest tube duration recorded for the NA group. Furthermore, the VATS learning curve could also have influenced this higher reintervention rate, although the year in which VATS was performed did not correlate with the complication rate in the multivariate analysis.
The results presented in this study can be further generalized for other teaching tertiary care institutions. The management of PE in our facility follows international guidelines, and the study population included all of the patients operated on during the study period (excluding the few patients whose medical charts could not be found). Therefore, our results reflect standard daily clinical practice, which is why we opted to include all consecutive PE cases, including those with different etiologies. However, the major threat to the generalization of these results is the fact that, due to particular features of the Brazilian public health system, many patients experience a lengthy preoperative time between their first admission to a health care facility and definitive surgical treatment. Referrals to a tertiary institution generally occur late in the context of this study, and this situation may be different at other facilities worldwide.
The main limitation of this study, apart from its retrospective design, was the potential bias associated with the analysis of different periods of time. To minimize this bias, we evaluated a period of only three years before and after the introduction of VATS. Moreover, no major changes to our postoperative care occurred throughout the study. The pain and respiratory therapy protocols were standardized prior to the period evaluated and, most importantly, the indications for surgical referral remained constant during the study period. Nevertheless, minor changes in antibiotic therapy and certain ICU routines occurred during the study period. In addition, the patients studied had been operated on by more than one surgeon, which may have interfered with our results. Finally, although the PE treatment algorithm was changed in 2005, we implemented this change gradually, as depicted in Figure 2, which could have led us to underestimate the potential differences between the two algorithms.
In conclusion, the incorporation of VATS into the algorithm for the treatment of PE did not significantly change the mortality or reoperation rates of the patients at our tertiary care hospital. Nevertheless, the more widespread use of VATS correlated with fewer postoperative complications and likely caused clinicians to send more severely ill patients to receive surgery.
AUTHOR CONTRIBUTIONSTerra RM designed the study and was responsible for the data analysis and manuscript preparation. Pêgo-Fernandes PM designed the study and was responsible for the data analysis and critical revision of the manuscript. Waisberg DR was responsible for the data collection and manuscript preparation. Almeida JL and Devido MS were responsible for the data collection. Jatene FB was responsible for critical revision of the manuscript.
No potential conflict of interest was reported.