During the neonatal and infancy periods, some chronic liver diseases may lead to progressive hepatic fibrosis, which is a condition that can ultimately result in the loss of organ function and severe portal hypertension necessitating hepatic transplantation. In a previous report, pharmacological interventions were demonstrated to modulate hepatic fibrosis induced by bile duct ligation in young rats. The administration of pentoxifylline or prednisolone, or the combination of both, resulted in reduced fibrogenesis in portal spaces. The objectives of the present study were to evaluate the expression of transforming growth factor β and vascular endothelial growth factor after bile duct ligation in young rats and to assess the effect of those same drugs on cytokine expression.
METHODS:In this experimental study, 80 young rats (21 or 22 days old) were submitted either to laparotomy and common bile duct ligation or to sham surgery. The animals were allocated into four groups according to surgical procedure, and the following treatments were administered: (1) common bile duct ligation + distilled water, (2) sham surgery + distilled water, (3) common bile duct ligation + pentoxifylline, or (4) common bile duct ligation + prednisolone. After 30 days, a hepatic fragment was collected from each animal for immunohistochemical analysis using monoclonal antibodies against transforming growth factor β and vascular endothelial growth factor. Digital morphometric and statistical analyses were performed.
RESULTS:The administration of pentoxifylline reduced the transforming growth factor β-marked area and the amount of transforming growth factor β expressed in liver tissue. This effect was not observed after the administration of prednisolone. There was a significant reduction in vascular endothelial growth factor expression after the administration of either drug compared with the non-treatment group.
CONCLUSIONS:The administration of pentoxifylline to cholestatic young rats resulted in the diminished expression of transforming growth factor β and vascular endothelial growth factor in liver tissue. The administration of steroids resulted in the diminished expression of vascular endothelial growth factor only. These pathways may be involved in hepatic fibrogenesis in young rats submitted to bile duct ligation and exposed to pentoxifylline or prednisolone.
Cholestatic disorders are responsible for chronic hepatic failure in a significant number of patients during infancy. In the neonatal period, the most frequent cholestatic disease requiring liver transplantation is biliary atresia, a condition for which the pathogenesis is not yet fully understood but that is absolutely fatal if left untreated (1).
Since the 1970s, some authors have suggested that the administration of corticosteroids may promote better late outcomes of biliary atresia in children who undergo Kasai's portoenterostomy (2-4). In recent years, some results of this new strategy have been published, but the available data still do not clearly demonstrate a difference in jaundice-free survival and a reduced need for early liver transplantation (5-7). Other potentially antifibrogenic drugs have also been experimentally tested in animals, including pentoxifylline (PTX), a phosphodiesterase inhibitor already used in clinical practice for the treatment of arterial insufficiency (8-10). Some studies have demonstrated the inhibition of inflammatory cytokines, such as tumor necrosis factor α, transforming growth factor β, and interleukins (ILs) 1, 6, and 8 (11,12). Other studies have failed to demonstrate this effect in animal models (9,13). Therefore, no consensus exists regarding the role of PTX in the inflammatory and fibrogenic cascades.
Our group has been investigating the effects of antifibrogenic drugs over the last few years, with a particular focus on their effects in growing animals. We reported our initial results in 2009 after developing a model to study portal fibrosis secondary to biliary obstruction in young rats (14-15). We concluded that hepatic fibrosis induced by bile duct ligation in young rats could be modulated by pharmacologic interventions. The administration of pentoxifylline or prednisolone (PRED), or the combination of both, resulted in diminished collagen-filled areas in the portal spaces. We have continued this work and now present an immunohistochemical analysis of the expression of transforming growth factor β (TGFβ) and vascular endothelial growth factor (VEGF) after bile duct ligation in young rats and the influence of these drugs on cytokine expression.
METHODSThis study was approved by the Ethical Committee for Research Project Analysis of our institution and was conducted according to international guidelines regarding the use of laboratory animals. All operative procedures were performed by the same surgeon.
Surgical proceduresYoung (21- to 22-day-old) Wistar rats were submitted to common bile duct ligation (CBDL) as described in a previous report (15). Under ether anesthesia, a median laparotomy was performed, and the common bile duct was ligated and divided twice using 6.0 monofilament nylon ligation. The sham surgery (SHAM) consisted of the laparotomy and exposure of the hepatic hilum without duct ligation. The animals were randomly allocated into four groups (20 animals per group) according to the surgical procedure and were administered a solution as follows: 1) CBDL + distilled water; 2) SHAM + distilled water; 3) CBDL + PTX 10 mg/kg per day; or 4) CBDL + PRED 3 mg/kg per day.
The solutions were administered once a day via nasogastric tubing under sedation. The drug doses per kilogram were similar to those used in humans in the clinical setting.
After 30 days, cardiorespiratory arrest was induced by the inhalation of anesthesia (ether gas chamber at a high concentration). The animals were rapidly weighed and submitted to midline incision for the harvesting of a 1-cm3 hepatic fragment from the left lobe.
Histological analysisThe hepatic fragments were processed according to standard techniques (3-μm-thick sections of paraffin-embedded material) to obtain two slides for each animal. The slides were included in 3-aminopropyl-trietoxi-silano, and immunohistochemical reactions followed the biotin-streptavidin-peroxidase protocol. The primary antibodies were as follows: monoclonal anti-mouse TGFβ IgG (sc-52893, Santa Cruz Biotechnology, USA) and monoclonal anti-mouse VEGF IgG (sc-7269, Santa Cruz Biotechnology, Santa Cruz, CA, USA). The secondary antibody was the Vectastain anti-mouse ABCkit™ (Vector Laboratories, Burlingame, CA, USA) for both reactions. The protein block was accomplished with methanol/azide for the TGFβ slides and with the Novocastra™ system (Leica Microsystems, Milton Keynes, England, UK) for the VEGF reactions. The slides were analyzed under a Leica DMR microscope and digitized with Panoramic Scan Midi™ (3DHistech, Hungary Software, Budapest, Hungary).
Morphometric analysisAleatory areas were delineated on each digitized slide and processed with the aid of the software Image Pro-Plus 4.5.0.29 Windows XP version (Media Cybernetics Inc.). After image acquisition, the software was calibrated to automate the cytokine (TGFβ or VEGF) identification. Several measurements were performed: total area, cytokine-marked area (area +), integrated optical density (IOD; antibody color intensity measure, which reflects the amount of cytokine present in the tissue), antibody distribution or proportion (area +/total area), and mean density (IOD/area +).
Statistical analysisComparisons of the data between groups were performed using the software SPSS version 18.0 for Windows (SPSS Inc., Chicago, IL). As the data had a normal distribution, the analysis of variance and Tukey’s post hoc test were applied to test for differences between groups. The hypothesis of sample equality was rejected for p<0.05.
RESULTSDescriptive analysisTGFβ expression
- 1.
Sham: no hepatocyte presentation, expression in endothelial and hepatic stellate cells;
- 2.
CBDL: mild expression in periportal hepatocytes, no expression in the biliary epithelium;
- 3.
PRED: irregular hepatocyte expression in different areas of the hepatic lobule, clear presentation in endothelial and hepatic stellate cells;
- 4.
PTX: less intense expression in hepatocytes and endothelial cells than in the PRED group.
VEGF expression: All groups exhibited VEGF expression in hepatocytes, endothelial and hepatic stellate cells and the biliary epithelium. Only fibrous areas were not stained (Figure 1).
Morphometric analysisTGFβ expressionValidating the findings of the descriptive analysis, the administration of pentoxifylline (PTX) to growing rats reduced the size of the cytokine-marked area (Figure 2) and the amount of TGFβ expressed in liver tissue (Figure 3), as reflected by the integrated optical density (IOD). These effects were not observed after the administration of prednisolone (Figures 2 and 3).
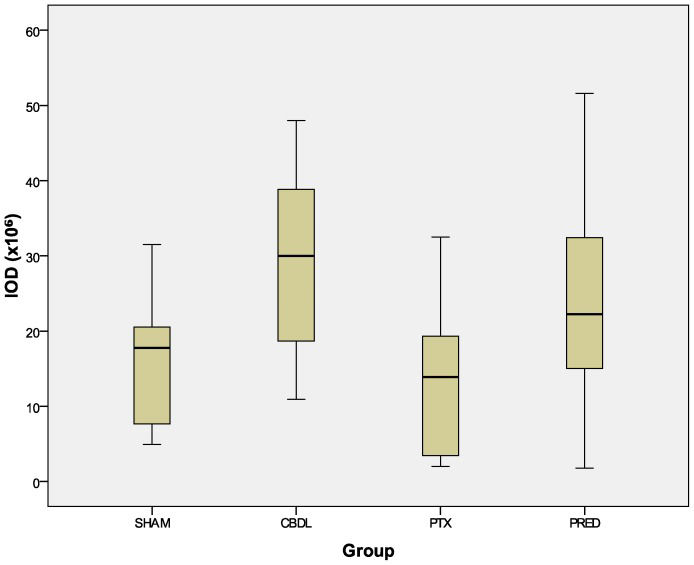
The reduction in the expression of VEGF after the administration of both drugs was quite remarkable. The distribution of this cytokine (Figure 4) was reduced in the PTX and PRED groups relative to the CBDL group (p<0.001). Although the decrease in the absolute amount of cytokine in the PRED group, as revealed by the IOD index, did not reach statistical significance (p = 0.195, Figure 5 compared with the CBDL group, when transformed into the mean density (ratio between the IOD and positive area), a significant decrease was revealed (p = 0.001, Figure 6.
Regarding the treatment of biliary atresia in children, clinicians have been empirically prescribing steroids immediately post-Kasai surgery since the 1980s (16-18). In the wake of progressive liver disease following bile duct obstruction, steroids are hypothesized to suppress inflammation and promote bile flow (19), but recent clinical reports have been controversial (20,21) and have failed to clearly demonstrate an actual long-term benefit (i.e., transplant-free survival) of the adjuvant use of steroids after portoenterostomy.
Another medication included in this study was pentoxifylline (PTX), a potentially antifibrogenic drug (9-13,12. Classified as a nonspecific phosphodiesterase inhibitor, this drug has already been incorporated into the clinical setting for the treatment of chronic occlusive arterial disease because of its oxygen transport-enhancing effect. As phosphodiesterases regulate the intracellular levels of cyclic nucleotides (cyclic adenosine monophosphate and cyclic guanosine monophosphate), their inhibition can affect several processes, such as apoptosis, muscle contraction, cellular differentiation, migration, and proliferation (23-25). Specifically regarding hepatic fibrogenesis, since 1993, there have been some publications demonstrating that PTX can decrease the activation and proliferation of stellate cells and downregulate the production of IL-1, IL-6, and tumor necrosis factor α (all proinflammatory cytokines) (26).
In a previous report (15), we revealed that the administration of a steroid and pentoxifylline to recently weaned rats submitted to biliary obstruction could promote a reduction in the collagen-filled areas in liver tissue, indicating a potential mechanism for the pharmacological modulation of cholestasis-induced portal fibrosis. We believe that the use of growing animals, rather than adults, as experimental models may more accurately represent the histological abnormalities found in newborns or children affected by cholestasis (14).
The exact mechanisms involved in portal biliary fibrosis in young animals are not completely understood. The present investigation was conducted to contribute to the elucidation of the following specific question: how is hepatic collagen deposition secondary to biliary obstruction affected by the administration of steroids and PTX?
Among other cytokines, TGFβ and VEGF appear to actively participate in the processes of inflammatory cell recruitment and hepatic fibrogenesis and the mediation of the adaptive proliferative response of cholangiocytes to cholestasis (27-29). Are these cytokines affected by the administration of steroids and PTX?
In this model, we were able to demonstrate, through immunohistochemical analyses, that VEGF expression was substantially reduced following exposure to steroids and PTX. However, only PTX appeared to affect the liver tissue expression of TGFβ. We failed to demonstrate a significant difference in this expression after the administration of prednisolone. The impairment in the pathways of these two cytokines after the administration of PTX to these growing animals may explain the slightly more accentuated reduction in collagen deposition observed in our previous report compared with the PRED group.
Obviously, other cells, cytokines, signaling pathways, and mechanisms are involved in a process as complex as hepatic fibrogenesis. Therefore, subsequent experimental and clinical studies should be conducted to confirm the benefits of exposing jaundiced children to these medications, with the aim of reducing hepatic fibrosis and the need for liver transplantation.
We thank FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) for providing financial support (grant #2009/07225-2).
No potential conflict of interest was reported.
Andrade WC designed and conceived the study, performed the experiments, conducted the analyses, and contributed to the discussion. Silva LF and Coelho MC conducted the analyses and contributed to the discussion. Tannuri AC, Alves VA, and Tannuri U contributed to the discussion.