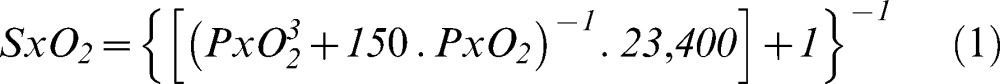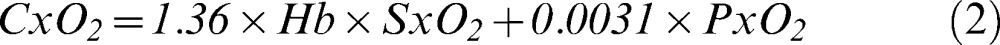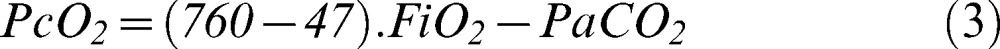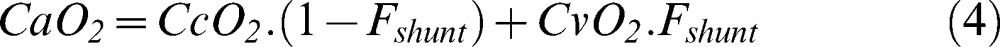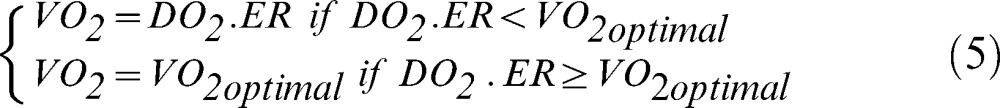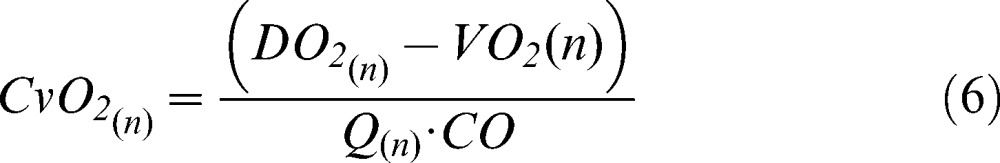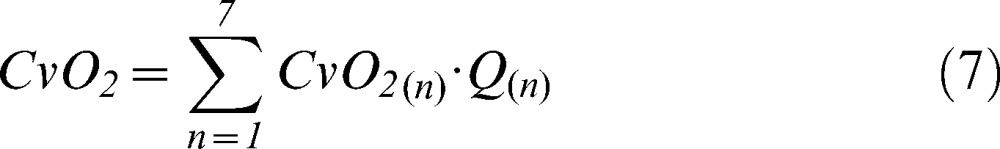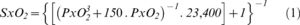Hemodynamic support is aimed at providing adequate O2 delivery to the tissues; most interventions target O2 delivery increase. Mixed venous O2 saturation is a frequently used parameter to evaluate the adequacy of O2 delivery.
METHODS:We describe a mathematical model to compare the effects of increasing O2 delivery on venous oxygen saturation through increases in the inspired O2 fraction versus increases in cardiac output. The model was created based on the lungs, which were divided into shunted and non-shunted areas, and on seven peripheral compartments, each with normal values of perfusion, optimal oxygen consumption, and critical O2 extraction rate. O2 delivery was increased by changing the inspired fraction of oxygen from 0.21 to 1.0 in steps of 0.1 under conditions of low (2.0 L.min-1) or normal (6.5 L.min-1) cardiac output. The same O2 delivery values were also obtained by maintaining a fixed O2 inspired fraction value of 0.21 while changing cardiac output.
RESULTS:Venous oxygen saturation was higher when produced through increases in inspired O2 fraction versus increases in cardiac output, even at the same O2 delivery and consumption values. Specifically, at high inspired O2 fractions, the measured O2 saturation values failed to detect conditions of low oxygen supply.
CONCLUSIONS:The mode of O2 delivery optimization, specifically increases in the fraction of inspired oxygen versus increases in cardiac output, can compromise the capability of the “venous O2 saturation” parameter to measure the adequacy of oxygen supply. Consequently, venous saturation at high inspired O2 fractions should be interpreted with caution.
The ultimate goal of hemodynamic support is to provide enough oxygen delivery (DO2) to guarantee tissue oxygen needs and avoid organ dysfunction (1,2). Under normal conditions, DO2 exceeds oxygen consumption (VO2) to an extent that makes consumption independent of delivery (3,4). However, during regional low delivery, oxygen consumption becomes supply dependent (3-5). Oxygen delivery depends on the hemoglobin concentration (Hb), hemoglobin oxygen affinity, arterial partial pressure of oxygen (PaO2), and cardiac output (CO). It is possible to achieve comparable changes in DO2 by manipulating these variables within physiological limits, although the impact on VO2 might depend on what parameter is modified (6).
Measuring oxygen consumption at the bedside is complex. Therefore, surrogates of the adequacy of oxygen delivery, such as lactate concentration, venous partial pressure of oxygen (PvO2), and mixed (SvO2) or central venous oxygen saturation, are commonly used during routine patient care (1,5,7). Venous oxygen saturation is part of the current guidelines for early resuscitation during sepsis (7). Nevertheless, SvO2 can respond differently to changes in DO2 if the changes are obtained through changes in different component of the DO2 equation (8), even when these changes lead to similar VO2 values. In this paper, we developed a mathematical model to investigate whether the type of DO2 optimization, specifically increases in the arterial partial pressure of oxygen versus increases in cardiac output, would have different effects on SvO2.
METHODSThe mathematical model was created based on the lungs and on seven peripheral compartments. Lung blood flow was divided into shunted and non-shunted areas. The peripheral compartments, each with its own perfusion, optimal VO2, and critical oxygen extraction rate, were created to simulate the behavior of the flow to organs. Having multiple peripheral compartments in the model was important to simulate the heterogeneity of the oxygen supply/demand in different organs and tissues to reproduce the complex relationship between mixed venous oxygen saturation and oxygen delivery.
In all of the following notations, “x” represents venous (“v”), lung capillary (“c”) or arterial blood (“a”).
Hemoglobin oxygen saturation (SxO2) was calculated according to equation 1 (9):
Blood oxygen content (CxO2) was calculated according to a standard formula (equation 2) (9):
Lungs: The alveolar partial pressure of oxygen was calculated using the alveolar gas equation (equation 3) and used as an approximation of the capillary partial pressure of oxygen (10):
As mentioned, the lungs were composed of shunted and non-shunted areas. Solving Berggren’s shunt equation for CaO2 gives the following equation (where Fshunt is the pulmonary shunt fraction):
DO2 and VO2 in the peripheral compartments: We used seven compartments to simulate blood flow to the brain, heart, kidneys, muscles, splanchnic (liver), skin, and 'others’, each with normal values of perfusion fraction, extraction rate, and optimal oxygen consumption (VO2optimal) (11). Because the arterial oxygen content was the same for all compartments, differences in oxygen supply from one compartment to the other occurred through differences in regional perfusion. For each compartment, the oxygen consumption was calculated according to equation 5:
The mathematical model: The input variables for the model included global values of cardiac output, pulmonary shunt fraction, pH, arterial carbon dioxide tension, Hb, and fraction of inspired oxygen. Additionally, we supplied hemodynamic variables for each compartment, including its perfusion fraction, its critical oxygen extraction rate and optimal VO2.
In the first run of the model, mixed venous oxygen saturation and mixed venous content were calculated according to equation 1 using an arbitrary initial mixed venous oxygen partial pressure of 40 mmHg. Pulmonary capillary oxygen content was obtained through equations 2 and 3, and arterial oxygen content was estimated according to the pulmonary shunt fraction (equation 4). Oxygen delivery to each compartment was subsequently calculated by multiplying the compartment perfusion fraction by the global oxygen delivery.
A new venous oxygen content value for each compartment was then calculated using equation 6, and the global mixed venous oxygen content was obtained from a perfusion-weighted average of the local venous contents (equation 7).
The mixed venous partial pressure of oxygen was determined from the mixed venous oxygen content by solving equations 1 and 2 to an acceptable error of 1:1,000 using the Newton-Raphson method. The new calculated value of the mixed venous partial pressure of oxygen was then reentered into the model, replacing the initial guess. We repeated these calculations until the difference between successive approximations of the mixed venous partial pressure of oxygen values was less than 1:1,000 of the previous value. The model outputted compartment and global final values of both the arterial and venous partial pressures of oxygen and saturation values.
The model was designed using The R Project for Statistical Computing (www.r-project.org) with the rootsolve package.
RESULTSIn all simulations, we maintained the following constant global values: Hb 14 g/dL, PaCO2 40 mmHg, pH 7.40, and a pulmonary shunt fraction of 0.1. Normal values of the perfusion fraction, which were critical for the oxygen extraction rate and optimal oxygen consumption, were also provided for each peripheral compartment (11).
We modeled two different states: normal-high and low oxygen delivery. For each of these states, we varied the oxygen delivery by changing the FiO2 with a fixed CO and changing the CO with a fixed FiO2. The first step was to obtain increasing DO2 values by changing the FiO2 from 0.21 to 1.00 in steps of 0.10 (with the exception of the first step = 0.09) with two values of cardiac output: 1) CO = 2.0 L.min-1 (low oxygen delivery) and 2) CO = 6.5 L.min-1 (normal-high oxygen delivery). In the next step, with a constant fraction of inspired oxygen (0.21), we chose cardiac output values to match the DO2 obtained in the previous step. In the normal-high delivery state, oxygen demands were always met (VO2≥197 mL.min-1), whereas in the low delivery state, the oxygen supplies were insufficient to meet the oxygen demands (VO2<197 mL.min-1).
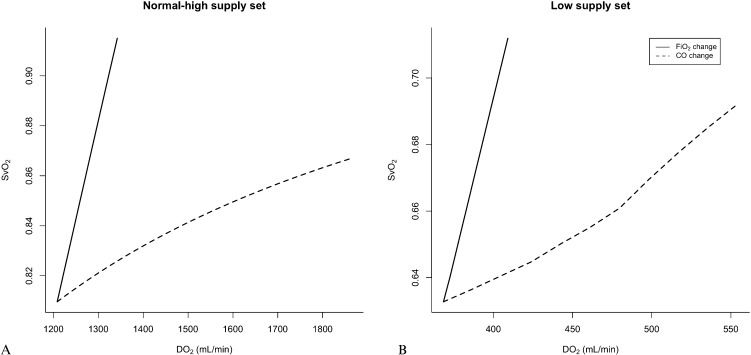
Normal-high supply set under this set of conditions, we simulated increases in DO2 under optimal oxygen consumption to all compartments (Table 1). The increase in CO caused a proportional increase in DO2, as expected. Conversely, DO2 could only be increased by 11% by nearly quintupling FiO2 from 0.21 to 1.0. This increase led to a change in SvO2 from 81 to 92%; in contrast, when the same increase in DO2 was accomplished by increasing CO, the SvO2 only increased from 81 to 83%. In fact, even a 50% increase in DO2 (Figure 1A) accomplished through augmenting CO alone only increased SvO2 to 86%. Of note, for this set of conditions, the peripheral compartments could have been easily lumped together with no loss of information because VO2 was constant among the different conditions simulated.
Effect of increasing the oxygen delivery on oxygen venous saturation by manipulating either the inspired fraction of oxygen or the cardiac output.
| Changes in FiO2 with fixed CO | Changes in CO with fixed FiO2 | |||||||
|---|---|---|---|---|---|---|---|---|
| DO2 | VO2 | FiO2 | CO | SvO2 | FiO2 | CO | SvO2 | |
| (mL.min-1) | (mL.min-1) | (L.min-1) | (%) | (L.min-1) | (%) | |||
| Normal-high supply set | 1,208 | 197 | 0.21 | 6.50 | 81 | 0.21 | 6.50 | 81 |
| 1,238 | 197 | 0.3 | 6.50 | 83 | 0.21 | 6.66 | 81 | |
| 1,256 | 197 | 0.4 | 6.50 | 85 | 0.21 | 6.75 | 82 | |
| 1,271 | 197 | 0.5 | 6.50 | 86 | 0.21 | 6.84 | 82 | |
| 1,286 | 197 | 0.6 | 6.50 | 87 | 0.21 | 6.91 | 82 | |
| 1,300 | 197 | 0.7 | 6.50 | 88 | 0.21 | 6.99 | 82 | |
| 1,315 | 197 | 0.8 | 6.50 | 89 | 0.21 | 7.07 | 82 | |
| 1,329 | 197 | 0.9 | 6.50 | 90 | 0.21 | 7.15 | 82 | |
| 1,344 | 197 | 1 | 6.50 | 92 | 0.21 | 7.22 | 83 | |
| Low supply set | 368 | 125 | 0.21 | 2.00 | 63 | 0.21 | 2.00 | 63 |
| 377 | 128 | 0.3 | 2.00 | 65 | 0.21 | 2.05 | 63 | |
| 383 | 129 | 0.4 | 2.00 | 66 | 0.21 | 2.08 | 64 | |
| 387 | 130 | 0.5 | 2.00 | 67 | 0.21 | 2.10 | 64 | |
| 392 | 131 | 0.6 | 2.00 | 68 | 0.21 | 2.13 | 64 | |
| 396 | 133 | 0.7 | 2.00 | 69 | 0.21 | 2.15 | 64 | |
| 401 | 134 | 0.8 | 2.00 | 70 | 0.21 | 2.18 | 64 | |
| 405 | 135 | 0.9 | 2.00 | 70 | 0.21 | 2.20 | 64 | |
| 410 | 136 | 1 | 2.00 | 71 | 0.21 | 2.22 | 64 | |
CO: cardiac output; DO2: oxygen delivery; FiO2: inspired fraction of oxygen; SvO2: venous oxygen saturation; VO2: oxygen consumption.
Panel A: Effect of increasing DO2 on the SvO2 through changes in the inspired fraction of oxygen (FiO2, solid line) or cardiac output (dashed line) under normal and high oxygen supply conditions sufficient to attain optimal oxygen consumption in all peripheral compartments. Panel B: Effect of increasing DO2 on the SvO2 through changes in the inspired fraction of oxygen (FiO2, solid line) or cardiac output (dashed line) under low oxygen supply conditions that caused the oxygen consumption to be supply limited.
When VO2 was supply limited, the relation between DO2 and SvO2 became more complex as a consequence of the critical extraction rates being reached at different DO2 values (Table 1 and Figure 1). Interestingly, the modest 11% increase in DO2 produced by augmenting FiO2 markedly increased SvO2 to values above 70%, masking the 31% deficit of VO2 present at an FiO2 of 1.0 (Table 1). In contrast, increasing DO2 by the same amount by improving CO led to an SvO2 of 64%, indicating insufficient oxygen supply.
DISCUSSIONIn this paper, we showed that when oxygen supply was increased by the same amount by increasing either FiO2 or CO, the resulting SvO2 was higher when DO2 was increased by manipulating FiO2. Of note, we found that under conditions of low oxygen supply, high values of the fraction of inspired oxygen could increase the mixed venous saturation to normal levels, masking states of insufficient oxygen supply.
Mixed venous saturation has long been used as a surrogate for adequate perfusion to tissues during resuscitation (5). Low levels of SvO2 are associated with poor prognosis for several clinical scenarios, and currently, guidelines suggest targeting SvO2 at 70% during the early phase of sepsis resuscitation, which is associated with a reduction in mortality (7). Nowhere in those guidelines, however, do the authors comment on the FiO2 at which the central venous blood gas should be collected.
One strength of the theoretical modeling approach is that it is possible to compare the effects of FiO2 and CO on the mixed venous saturation at precisely the same oxygen supply/demand conditions, i.e., equal VO2 and DO2. Ho et al. have previously shown that in humans with shock, high levels of PaO2 can increase SvO2 levels (8). From their data, however, it was not possible to determine whether the increase in SvO2 was indeed reflecting a better oxygen supply/demand relationship or whether the SvO2 represented a false marker of the adequacy of such a relationship. Our findings of increases in SvO2 to levels considered normal caused by increasing the FiO2 in the setting of insufficient oxygen supply support the latter hypothesis.
The mechanism by which increased values of FiO2 cause a steeper increase in SvO2 than that caused by increases in CO can be explained by the conservation of mass. When manipulating the FiO2 at fixed values of CO and oxygen consumption, the arteriovenous difference in oxygen content (CaO2 – CvO2) must remain constant because oxygen consumption is equal to the product of cardiac output and the arteriovenous difference in oxygen content. Thus, in this scenario, increases in arterial oxygen content will be followed by equal increases in venous oxygen content, explaining the roughly linear relationship between SvO2 and DO2 shown in Figure 1. Conversely, when manipulating CO while keeping arterial oxygen content and oxygen consumption constant, the increase in CO will be compensated for by an increase in the venous oxygen content proportional to the arteriovenous difference in oxygen content such that at high CO values, the venous oxygen content approaches the arterial oxygen content. For example, using the Fick principle, if one assumes a hypothetical initial arterial oxygen content of 16 mL/dL and a venous oxygen content of 12 mL/dL, a 25% increase in the DO2 by increasing the arterial oxygen content to 20 mL/dL will cause an increase in venous oxygen content by the same amount (from 12 to 16 mL/dL), causing venous saturation to increase from 62 to 83%. The same 25% increase in DO2 caused by increasing CO will only decrease the arteriovenous difference in oxygen content from 4.0 to 3.2 mL/dL, thus increasing the venous oxygen content from 12 to 12.8 mL/dL. The result would be a modest increase in the central venous oxygen saturation from 62 to 66%.
Our study has several limitations. We kept the values of pH and the arterial partial pressure of carbon dioxide, variables known to be affected by the hemodynamic condition, constant during all simulations. Although the model could accommodate changes in these variables, we decided to keep them constant to isolate the effects of the fraction of inspired oxygen and cardiac output. Additionally, for similar reasons, we ignored potential changes in the perfusion fraction that could be caused by the local control of blood flow during regional hypoxemia. Blood flow to tissues is usually driven by local metabolic demands to match supply and demand. Therefore, this model does not take into account changes in the local oxygen consumption that may occur after changes in the local partial pressures of oxygen and carbon dioxide.
In conclusion, we showed that the type of DO2 optimization, specifically increases in FiO2 versus increases in CO, could affect the capability of venous oxygen saturation to measure the adequacy of oxygen supply. Interpreting venous saturation at high arterial partial pressures of oxygen values should be performed with caution.
No potential conflict of interest was reported.
Zampieri FG wrote the manuscript, performed simulations with the model and wrote the mathematical model. Park M revised the manuscript and helped with model construction. Azevedo LCP performed simulations with the model and revised the manuscript. Amato MBP revised the manuscript and helped with model construction. Costa EDL designed and wrote the mathematical model and helped with manuscript preparation.