This study investigated the effects of buflomedil and pentoxifylline, both of which are used in reconstructive surgery of hamster skin flap microcirculation, and evaluated the skin flap survival rate by orthogonal polarization spectral imaging.
METHODTwenty-four adult male Syrian golden hamsters were divided into three groups: a control (C, 0.1 ml 0.9% saline), buflomedil (B, 3 mg/kg/day), and pentoxifylline group (P, 14.5 mg/kg/day). Treatments administered intraperitoneally were initiated 1 hour before skin flap preparation and continued for 7 days post-operatively at 12-hour intervals. Preparations (skin flaps) were divided into 12 fields, which were organized into six bands. Functional capillary density (FCD, in mm/mm2), distance from the skin flap base to blood flow cessation (Distwith flow, in cm), percentage of viable skin (VA, in%), and qualitative analysis of blood flow by orthogonal polarization spectral imaging were performed at 1 and 24 hours and on the seventh post-operative day.
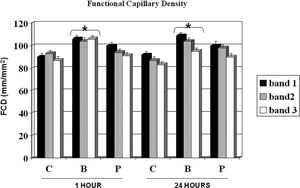
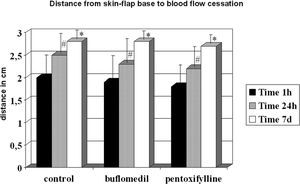
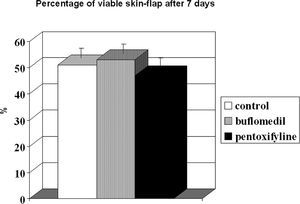
RESULTBands IV, V, and VI presented no flow independent of time. The functional capillary density group B was higher than that of groups C and P, primarily after 24 hours. All groups showed an increase in D with time but reached similar final distances (C = 2.73, B = 2.78 and P = 2.70 cm). Moreover, the percentage of viable areas remained at approximately 50%. The orthogonal polarization spectral imaging was useful to assess viability by counting fields with and without blood flow.
CONCLUSIONSFunctional capillary density values were higher in the buflomedil group compared to the control and pentoxifylline groups in this model. Functional capillary density did not influence D or the percentage of VA, and the technique showed favorable potential to assess/predict the viability of skin flaps within 1 h after surgery.
Skin flap necrosis is a serious concern in reconstructive surgery. The surgical procedure used to create flaps involves tissue damage because some areas may exceed the territory supplied by a given blood vessel, leading to several degrees of ischemia.1 Moreover, microvascular perfusion is seriously impaired with failing procedures.2,3 Vasoactive drugs can be used to ameliorate the ischemia process and extend survival. Buflomedil and pentoxifylline have often been used in the treatment of peripheral artery disease and microcirculatory deficit, resulting in improved flap viability.4–7
Buflomedil is an alpha-receptor blocking agent and a non-specific calcium antagonist. It can also inhibit platelet aggregation, increase red blood cell deformability, and decrease peripheral vascular resistance, thus restoring microcirculatory perfusion.8 Buflomedil appears to improve nutritional blood flow in ischemic tissue in peripheral or cerebral diseases. Consequently, it increases walking distances in patients suffering from intermittent claudication.9 Pentoxifylline is a xanthine-derived phosphodiesterase inhibitor that enhances cAMP levels, theoretically via the same actions as buflomedil,10 i.e., it has beneficial effects on white blood cell function and hemorheological conditions.11 The effects of buflomedil and pentoxifylline administration on functional capillary density, tissue viability based on blood flow presence/absence, and survival of skin flaps in hamsters have yet to be elucidated. Skin flap microcirculation can be evaluated by orthogonal polarization spectral (OPS) imaging. This recently developed microscopy method, originally described by Winkelman,12 can be used to non-invasively study the microcirculation with reflected light without any fluorescent dye.13 Olivier and co-workers showed that OPS technology is useful to directly observe microvascular blood flow in skin flaps and suggested post-operative monitoring of free-tissue transfers.14 The OPS technique has been incorporated into a portable device, the Cytoscan (Cytoscan, Cytometrics Inc., Philadelphia, PA, USA).
This study tested the efficiency of OPS imaging for the prognosis of flap viability, and compared the effects of acutely administered buflomedil and pentoxifylline on the microcirculation using a random-pattern skin flap survival test.
MATERIALS AND METHODSExperiments were performed according to the protocols approved by the Ethical Committee of the State University of Rio de Janeiro, RJ, Brazil (CEA/215/2007) using 24 male 10-week-old Syrian golden hamsters (Mesocricetus auratus, Botucatu, São Paulo, SP, Brazil) weighing 110–130 g. Hamsters were divided into three groups. The control group received 0.1 ml of vehicle (C, 0.9% NaCl), the buflomedil (B) group received 3 mg/kg/day of buflomedil (Abbott, São Paulo, Brazil), and the pentoxifylline (P) group received 14.5 mg/kg/day of pentoxifylline (Aventis Pharma, São Paulo, Brazil). All injections were administered intraperitoneally 1 hour before skin flap preparation and for 7 days post-operatively at 12-hour intervals. Animals received an appropriate laboratory diet of Nuvital (Nuvilab, Curitiba, PR, Brazil) and water provided ad libitum. Animals were anesthetized with 30 mg/kg of sodium pentobarbital (Pentobarbital sodique, Sanofi, Paris, France, 60 mg/ml) intraperitoneally. Throughout the surgery and subsequent experiment, the temperature of the animals was maintained at 36.5 °C with a heating pad controlled by a rectal thermistor (LTB 750 Thermostat System, Uppsala, Sweden). A dorsal tricotomy was performed, followed by local antisepsia with 70% alcohol. The dorsal flap was dissected, preserving the sacral arteries, and detached from its panniculus carnosus. The area was subsequently sutured to the dorsal region of the animal using simple stitches. The surgical protocol was based on the McFarlane skin flap model (Olivier et al.)14,15 adapted to hamsters.
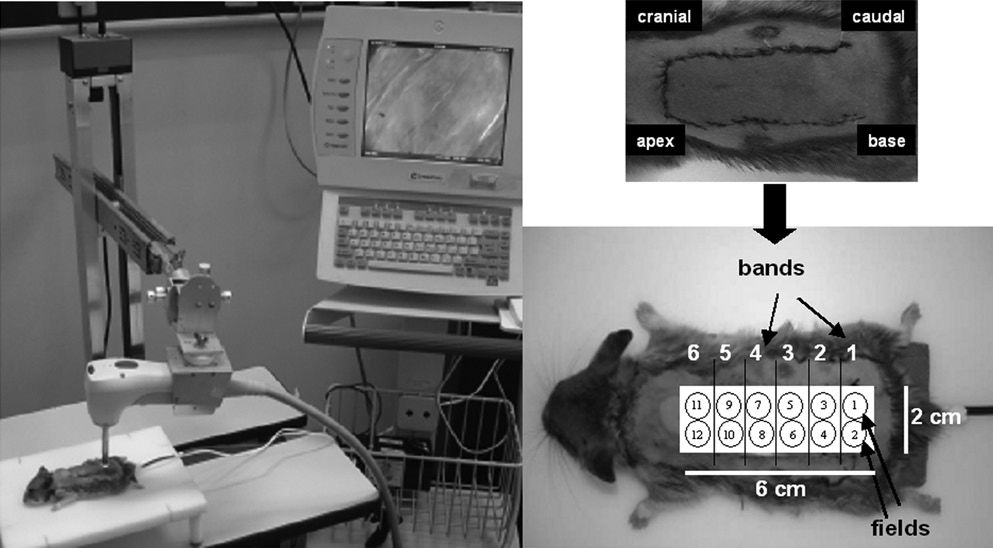
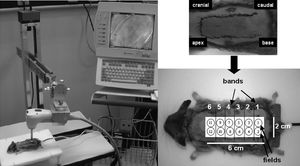
OPS imaging ProtocolEach dorsal randomized skin flap measured 2 x 6 cm and was divided into 12 fields of 1 cm in diameter. Each of the two fields formed one band to be analyzed (Figure 1). An OPS probe, with a 10x objective and a final magnification of 450x, was applied to these fields from the base of the flap to the apex (Figure 1). The center of each field had a microscopic area of 700×550 μm. Images of the microcirculation were video recorded, stored in VHS format, and subsequently processed by CapImage.16 Sterile mineral oil was applied between the probe and skin to improve the coupling and reliability of the readings.
Parameters EvaluatedFunctional Capillary Density (FCD)Functional capillary density (FCD) was defined as the total length of capillaries with flowing red blood cells in the microscopic area (mm/mm2) under observation. This was measured at 1 and 24 hours after the surgical procedure. The mean values of the fields from each band are reported.
Distance from the skin flap base to blood flow cessation (Distwith flow)The distance from the skin flap base to the blood flow cessation (Distwith flow) field was measured at 1 h, 24 h, and 7 days after surgery and compared to viable skin flaps.
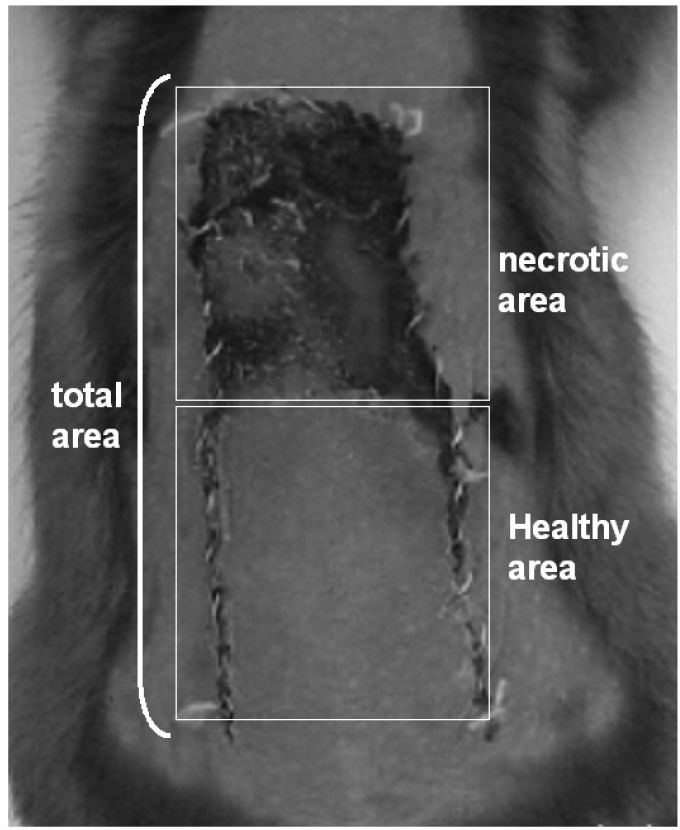
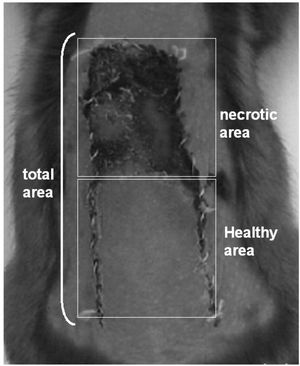
Percentage of viable skin flap area (%VA)A transparent sheet was placed on the dorsal skin flap and drawings of the areas with blood flow and necrosis were performed. The percentage of viable area (%VA) was calculated by dividing the healthy area by the total area on the seventh post-operative day using Autocad 2000 (Autodesk, San Rafael, USA) (Figure 2).
Qualitative analysis of blood flow by OPS imagingThe presence or absence of blood flow in the studied skin flaps was quantified in all 12 fields, grouped in bands (Figure 1) after 1 and 24 h. The mean values of the fields from each band are reported. The data obtained were compared to the blood flow in the viable area observed on the seventh postoperative day.
Statistical AnalysesFor clarity, the results are presented as the mean ± standard error of the mean (S.E.M.). For statistical analysis, Mann-Whitney U tests, ANOVAs, and Wilcoxon matched pair tests were used to compare groups, and P-values < 0.05 were considered as statistically significant.
RESULTSAll animals investigated did not demonstrate blood flow after the third band of the skin flap, independent of the postoperative time.
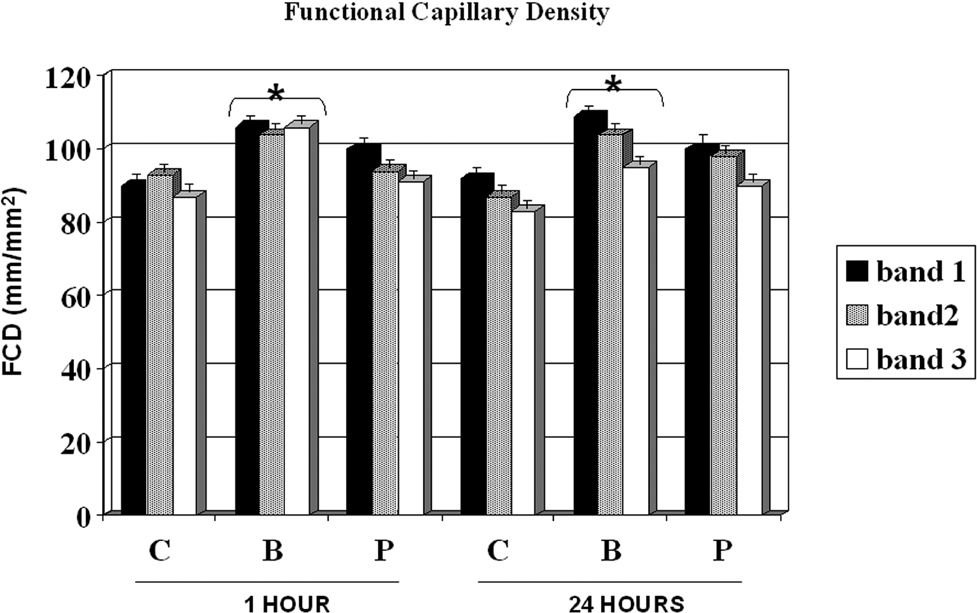
Figure 3 shows the FCD for the control, buflomedil, and pentoxifylline groups on bands I, II, and III 1 and 24 hours after the surgical procedure. A decrease in FCD was observed from band I to band III in all groups analyzed after 24 hours. The buflomedil group showed higher FCD values compared to the control or pentoxifylline group for each band at 1 and 24 h. Conversely, no statistical differences were observed for Distwith flow between the control, buflomedil, and pentoxifylline groups.
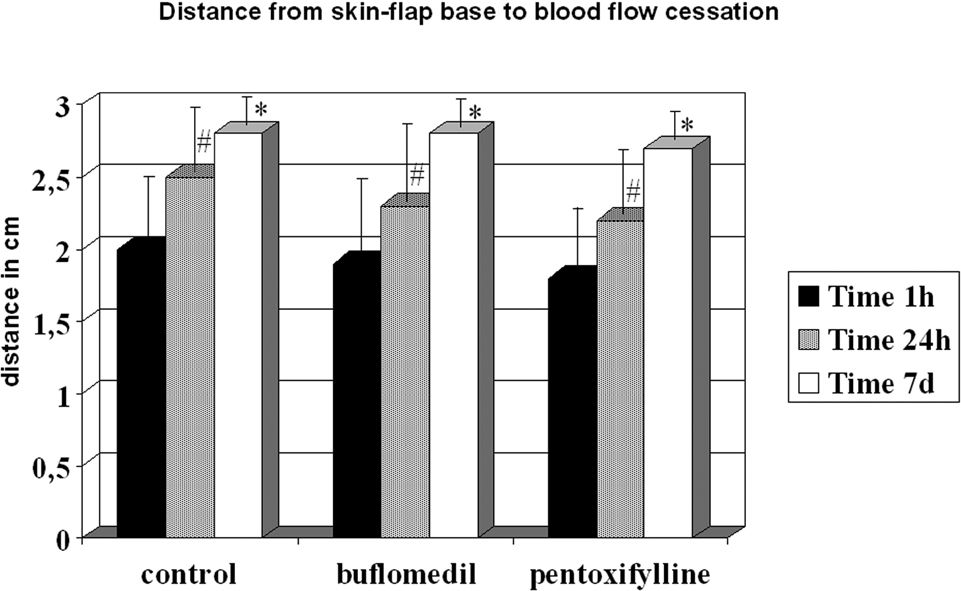
Although all groups displayed increased distances between 24 h and the seventh day, they reached similar final distances (control = 2.73 cm, buflomedil = 2.78 cm, and pentoxifylline = 2.70 cm) (Figure 4).
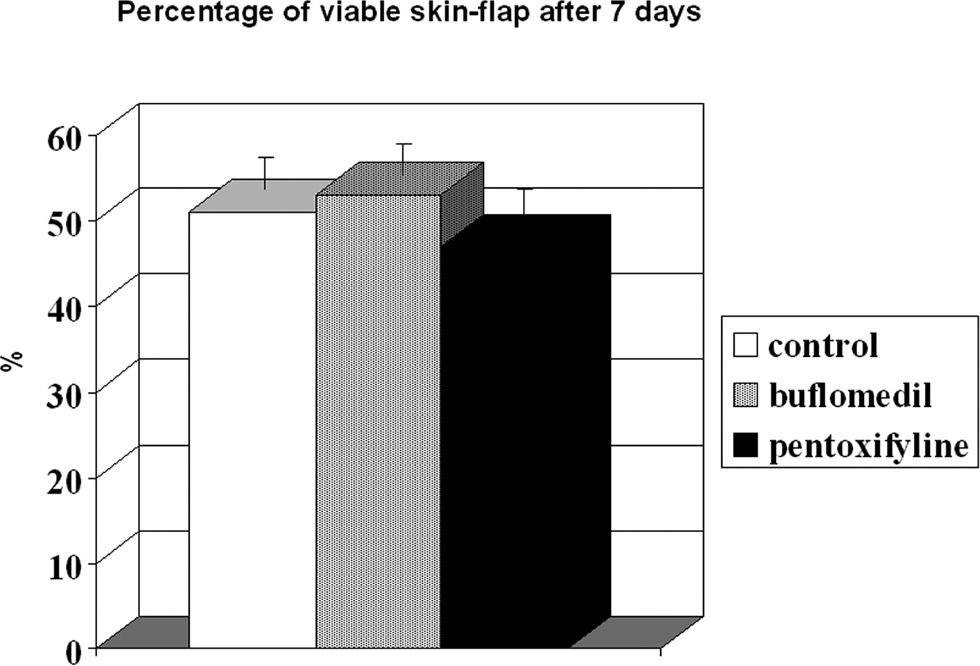
The percentage of viable area is illustrated in Figure 5. The perfusion/non-perfusion interface was easily identified, and there was no difference in the percentage of viable areas among the control, buflomedil, and pentoxifylline groups (50.7, 52.9, and 47.0, respectively). Finally, the mean percentage of the preserved area was approximately 50% for all groups.
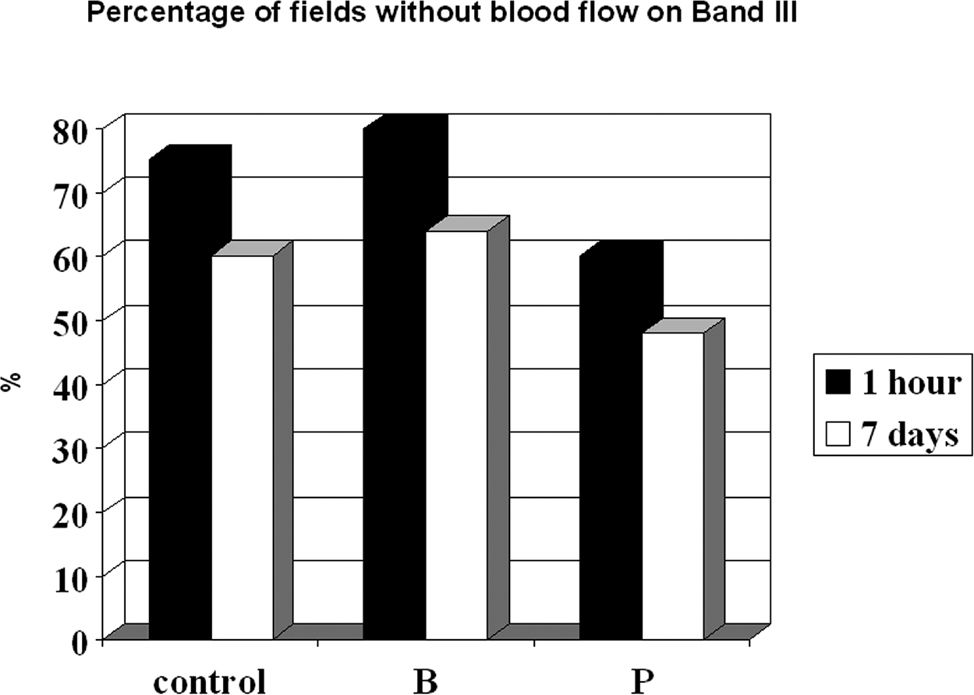
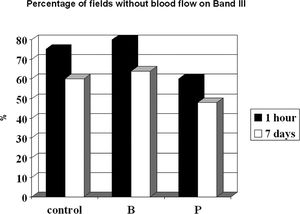
On the seventh post-operative day, we were able to confirm the presence of blood flow, which was detected after 1 h using OPS imaging of the first three bands for all groups. Using this technique, however, we were unable to correlate all areas that lacked blood flow with a given time line. For band II, an area of 100% in the control, buflomedil, and pentoxifylline groups without blood flow 1 h after surgery developed into perfused zones 7 days after surgery. The prediction of blood flow absence (in %) after 1 h and on postoperative day 7 for the control, buflomedil, and pentoxifylline groups for band III is shown in Figure 6. Note that the percentage of fields without blood flow detected by Cytoscan in controls decreased from 75% after 1 h to 60% after 7 days. This corresponds to a prediction power of 80%. The same pattern occurred for the buflomedil and pentoxifylline groups.
DISCUSSIONWhen a skin flap is raised, injury to the blood vessels and sympathetic nerves causes hemodynamic instability, leading to blood flow abnormality and a disturbance in tissue oxygenation.17 In this work, two approaches were used to evaluate skin flap success. We used buflomedil and pentoxifylline and determined their effects on the microcirculation and skin flap survival (FCD, Distwith flow, %VA), and the OPS imaging technique to predict flap viability over time following surgery.
Since significant microvascular damage is present during skin flap preparation, the development of early necrotic areas is expected. Indeed, all fields on bands IV, V, and VI did not present any blood flow, resulting in tissue death. The FCD decreased in all groups from band I to band III and was significantly lower on the distal part of the flap compared to its base after 24 h. The buflomedil group demonstrated higher values of FCD compared to the control and pentoxifylline groups. These results are consistent with the results reported by Langer and colleagues, who validated the use of OPS imaging against intravital microscopy and measured FCD on the skin flaps of hairless mice.18 Harder et al. also showed a decrement in FCD in several segments from the base to the apex of the skin flaps of mice.19 Buflomedil has been shown to have positive effects on microvascular reperfusion injury by decreasing leukocyte adhesion.20 However, Szczesny et al. showed that buflomedil is ineffective in avoiding a decrease in the FCD of local skin blood perfusion during general anesthesia using intravital fluorescence microscopy in hairless mice.21 In addition, resting skin flux motion activity at the hallux in patients with intermittent claudication evaluated by laser Doppler fluxometry was not increased after buflomedil treatment.22 It is possible that the results presented herein could be explained by better buflomedil effects on the redistribution of blood flow after ischemia compared to pentoxifylline. In fact, Briguglio et al. demonstrated an attenuation of ischemia-induced histological loss and damage to pyramidal cells in rats treated with buflomedil.23 On the other hand, FDC showed favorable results and Distwith flow did not change among the control, buflomedil, and pentoxifylline groups at any time point analyzed. For each group, increasing values of Distwith flow were detected from 1 hour to 7 days. Conflicting results have been reported concerning the actions of buflomedil and pentoxifylline on surface length necrosis on flaps or microcirculation. Quirinia and colleagues did not find any differences in wound healing for buflomedil compared to the control (no treatment) in rats24; however, Kamler and et al. showed an acceleration of wound surface area reduction after chronic ischemia.25 On the other hand, pentoxifylline elicited improved cardiovascular autonomic function, but failed to reduce microalbuminuria in type 2 diabetic patients, revealing a microcirculatory defect.26
The percentage of viable area on the seventh day after the surgical procedure did not differ among the groups studied and remained at approximately 50%, consistent with the buflomedil results described by Biondo-Simões et al.27 This result could be explained by the following scenarios. The treatment duration was not sufficient to produce favorable blood flow redistribution (treatment was initiated 1 h before surgery and lasted 7 days), allowing only acute effects to be detected. Bayat and colleagues chronically pretreated rats for 30 days with pentoxifylline before skin flap preparation and observed an increase in flap survival.28 Emrecan et al. showed that pentoxifylline could have an acute effect on the skeletal muscle of rabbits in an ischemia-reperfusion model.29 Our group selected buflomedil (3 mg/kg/day) and pentoxifylline (14.5 mg/kg/day) administration for 7 days post-surgery to elucidate the acute drug effects.
Another possibility could be that buflomedil and pentoxifylline are not capable of improving the microcirculation without any previous impairment of this structure. A lower percentage of viable skin flap areas in pretreated rats has been demonstrated with nicotine, which decreases blood flow to peripheral vessels by 40%. The percentage of viable areas was larger for the buflomedil and pentoxifylline groups than for rats administered nicotine.30 Finally, severe impairment of the microcirculatory network during the surgical procedure might interfere with drug action. It is well known that thin skin flaps may jeopardize the circulation.31 In the present study, the dorsal skin flap and sacral arteries containing the panniculus carnosus were preserved. It is likely, however, that some degree of arteriovenous shunting occurred over 24 h in the early and/or late post-operative periods, thereby limiting the skin survival in areas with marginal blood flow.32 Using a pig skin flap model, Kerrigan et al. observed that the dermal vessels of an ischemic island flap were dramatically affected.3 We also observed several distal random ischemic regions, which may have determined the final survival of the flap.
The Cytoscan was very effective in predicting skin flap viability. All fields in which blood flow was present after 1 and 24 h (detected by Cytoscan) remained viable 7 days after surgery.
Conversely, the prediction rate for fields without blood flow was not as effective. All fields without blood flow on band II and 20% of the fields on band III in all groups recovered after 7 days. Thus, the Cytoscan was more efficient in predicting fields without blood flow on band III, which had direct contact with the necrotic area and was subjected to unstable hemodynamics. It must be noted that some fields with unpredictable blood flow recovery will normally be present,33,34 and it should be emphasized that the OPS technique appeared to be more accurate than the clinical evaluation of skin flap necrosis.14 Other evaluations of microcirculation techniques also exist, such as intravital fluorescence microscopy,35 but the portability of this technique is limited. Laser Doppler flowmetry36 also appears to be a promising technique for use with OPS imaging.
CONCLUSIONSBuflomedil presented higher values of FCD compared to the control and pentoxifylline groups in the skin flap hamster model. However, no differences were observed for the distance from the skin flap base to blood flow cessation or the percentage of viable skin flaps among groups.
Orthogonal polarization spectral imaging is a very useful tool to assess skin flap viability by counting fields with and without blood flow, showing the ability to confirm 100% of the areas with and 80% of the areas without flow over time.
The authors wish to thank Mrs. Cristiane Maria Simonato Conde and Miss Kelly Silva de Andrade for technical support.