The coronavirus disease (COVID-19) outbreak has catastrophically threatened public health worldwide and presented great challenges for clinicians. To date, no specific drugs are available against severe acute respiratory syndrome coronavirus 2. Mesenchymal stem cells (MSCs) appear to be a promising cell therapy owing to their potent modulatory effects on reducing and healing inflammation-induced lung and other tissue injuries. The present pilot study aimed to explore the therapeutic potential and safety of MSCs isolated from healthy cord tissues in the treatment of patients with COVID-19.
METHODS:Twelve patients with COVID-19 treated with MSCs plus conventional therapy and 13 treated with conventional therapy alone (control) were included. The efficacy of MSC infusion was evaluated by changes in oxygenation index, clinical chemistry and hematology tests, immunoglobulin (Ig) levels, and pulmonary computerized tomography (CT) imaging. The safety of MSC infusion was evaluated based on the occurrence of allergic reactions and serious adverse events.
RESULTS:The MSC-treated group demonstrated significantly improved oxygenation index. The area of pulmonary inflammation decreased significantly, and the CT number in the inflammatory area tended to be restored. Decreased IgM levels were also observed after MSC therapy. Laboratory biomarker levels at baseline and after therapy showed no significant changes in either the MSC-treated or control group.
CONCLUSION:Intravenous infusion of MSCs in patients with COVID-19 was effective and well tolerated. Further studies involving a large cohort or randomized controlled trials are warranted.
The end of 2019 witnessed the emergence of coronavirus disease (COVID-19), an outbreak caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The highly contagious virus spread worldwide, resulting in a pandemic (1).
According to the World Health Organization (1), while most people with COVID-19 develop asymptomatic or mild illness, approximately 14% develop severe disease requiring hospitalization and oxygen support, and 5% require admission to the intensive care unit (ICU) on ventilation. For the treatment of COVID-19, most existing preclinical and clinical data on antiviral therapy are derived from other viruses, such as the coronavirus responsible for severe acute respiratory syndrome. However, it is unclear how well these data can be extrapolated to SARS-CoV-2. Viral vaccines are still under development and investigation. Thus, optimized supportive care remains the mainstay of therapy.
Severe cases of COVID-19 are often associated with rapid viral replication and considerable inflammatory cell infiltration (2,3). Autopsy studies on patients with COVID-19 have suggested that a dysregulated immune response occurs, resulting in excessive inflammation (cytokine storm) and lethal acute respiratory distress syndrome (ARDS) (2). Mesenchymal stem cells (MSCs) appear to be a promising cell therapy because they favorably modulate the immune response to reduce lung injury (4,5).
In preclinical models, MSCs have been shown to restore alveolar epithelial and endothelial permeability and to enhance the resolution of acute lung injury (ALI)/ARDS by secreting angiopoietin and keratinocyte growth factor (6). The clinical potential of MSCs to treat ALI/ARDS was shown to be considerably enhanced in a recent study using an ex vivo-perfused human lung preparation model (7). Treatment with allogeneic human MSCs (hMSCs) following endotoxin-induced lung injury reduced extravascular lung water, improved lung endothelial barrier permeability, and restored alveolar fluid clearance.
Based on these promising preclinical data, we conducted an MSC-transplantation pilot study in early March 2020 to explore its therapeutic potential and safety in treating patients with COVID-19.
METHODSStudy design and patientsThis pilot study included patients treated with MSCs plus conventional therapy and those treated with conventional therapy alone (controls). From February 10 to March 30, 2020, the medical team supporting Hubei province from the Second Hospital of Shandong University took over Wards E1-7, Optics Valley District, Tongji Hospital Affiliated to Tongji Medical College of HUST, Wuhan, China, to treat patients with relatively severe COVID-19. COVID-19 was diagnosed by positive throat-swab samples, which were detected using real-time reverse transcription-polymerase chain reaction (RT-PCR) assays. The detailed RT-PCR process has been described in previously published literature (8). Patients were judged to be in a moderate, severe, or critical stage according to the protocol released by the National Health Commission of the People’s Republic of China (9,10). Patients with moderate disease were characterized by fever, respiratory system symptoms, and pneumonia imaging findings. Patients with severe disease were characterized by respiratory distress (respiratory rate more than or equal to 30 times per minute), blood oxygen saturation at rest less than 93%, or an oxygenation index equal to or less than 300 mmHg. Patients with critical disease were characterized by respiratory failure that required mechanical ventilation, shock, failure of other organ(s), or the need for treatment in the ICU (9,10).
The protocol of the current study was performed in accordance with the principles of the Declaration of Helsinki. This study was approved by the Ethical Committee of Tongji Hospital Affiliated to Tongji Medical College of HUST and the Second Hospital of Shandong University (approval no. KYLL-2020(LW)-033). Patients who consented to MSC infusion were included in the MSC-treated group, while patients who consented to the use of clinical data were retrospectively included in the control group.
MSC preparation and infusionThe hMSCs were isolated and expanded from human umbilical cord samples and further characterized. These cells did not express hematopoietic lineage markers, such as CD34, CD45, and human leucocyte antigen DR, and were positive for CD44, CD73, CD90, and CD105, which demonstrated a characteristic immunophenotype of hMSCs. In addition, their multilineage differentiation ability (into osteogenic, adipogenic, and chondrogenic lineages) was demonstrated. The cell product was certified by the National Institutes for Food and Drug Control of China (authorization sample number: SH0417201907019). Clinical grade MSCs were supplied for free by Shandong Qilu Cell Therapy Engineering Technology Co., Ltd., a subsidiary company of Shandong Yinfeng Biological Group. MSCs were suspended in 100 mL normal saline, and the total number of transplanted cells was calculated as 1×106 cells/kg. The cells were infused at ∼60 drops per minute for approximately 30 min.
Data collectionMSC-infusion efficacy was evaluated by changes in oxygenation index, clinical chemistry and hematology tests, immunoglobulin (Ig) levels, and pulmonary computerized tomography (CT) imaging. The following clinical chemistry and hematology tests at baseline and during treatment were recorded: white blood cell count (WBC), neutrophil count (Neu), lymphocyte count (Lym), blood urea nitrogen (BUN), creatinine (Cr), D-dimer, N-terminal pronatriuretic peptide, troponin I, C-reactive protein (CRP), interleukin (IL)-6, IL-10, and tumor necrosis factor alpha (TNF)-α. Data on the clinical characteristics of the included patients were also collected.
Allergic reactions and serious adverse events (SAEs) were recorded to evaluate the safety of MSC infusion.
Statistical analysisIBM SPSS (version 22.0; IBM Corp., Armonk, NY, USA) was used to perform the following statistical analyses. Continuous variables are presented as means±standard deviations or medians (interquartile ranges [IQRs]). Categorical variables are presented as numbers and percentages. Data were compared using the Mann-Whitney U test, Wilcoxon rank sum test, one-way analysis of variance, least significant difference test, or Kruskal-Wallis test, as appropriate. Statistical significance was set at p<0.05.
RESULTSBaseline characteristicsMSC infusion was administered to 12 patients. The median age was 67 years (IQR: 56-70 years), and seven patients (58.3%) were men. One patient was considered critical upon admission. The median interval time from disease onset to MSC infusion and from admission to MSC infusion was 42 days (IQR: 29-46 days) and 18 days (IQR: 9-27 days), respectively. The mean infusion cell number was 6.3±0.8×107 (Table 1).
Baseline clinical characteristics of patients receiving mesenchymal stem cell therapy.
| Oxygen support | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Age (years) | sex | comorbidities | Clinical classification | Days from disease onset to MSC application | Infusion volume (107) | Antiviral therapy | Use of methylprednisolone | Before MSC | Two weeks after MSC |
| 1 | 72 | male | diabetes mellitus | severe | 69 | 6.00 | Arbidol, lopinavir-ritonavir | no | low-flow nasal cannula | none |
| 2 | 39 | male | none | severe | 32 | 8.00 | Arbidol, lopinavir-ritonavir | no | low-flow nasal cannula | none |
| 3 | 66 | male | hemorrhagic cerebral infarction | severe | 19 | 6.00 | Arbidol, lopinavir-ritonavir | no | low-flow nasal cannula | none |
| 4 | 63 | female | none | severe | 31 | 6.00 | Arbidol, lopinavir-ritonavir | yes | high-flow nasal cannula | high-flow nasal cannula |
| 5 | 82 | female | none | severe | 29 | 5.40 | Arbidol, lopinavir-ritonavir | yes | low-flow nasal cannula | low-flow nasal cannula |
| 6 | 68 | male | none | moderate | 46 | 6.70 | Arbidol, lopinavir-ritonavir | no | low-flow nasal cannula | none |
| 7 | 69 | male | none | critical | Not available | 7.00 | Arbidol, lopinavir-ritonavir | yes | mechanical ventilation | mechanical ventilation |
| 8 | 49 | male | none | moderate | 45 | 6.00 | Arbidol, lopinavir-ritonavir | no | low-flow nasal cannula | none |
| 9 | 53 | female | none | moderate | 42 | 6.00 | Arbidol, lopinavir-ritonavir | no | low-flow nasal cannula | none |
| 10 | 64 | female | none | severe | 28 | 5.20 | Arbidol, lopinavir-ritonavir | yes | low-flow nasal cannula | none |
| 11 | 70 | male | none | moderate | 44 | 6.30 | Arbidol, lopinavir-ritonavir | no | low-flow nasal cannula | none |
| 12 | 67 | female | none | moderate | 48 | 7.20 | Arbidol, lopinavir-ritonavir | no | low-flow nasal cannula | none |
MSC, mesenchymal stem cell.
A total of 13 patients who received conventional therapy were selected as the control group, with a median age of 68 years (IQR: 65-78 years), and five of the 13 patients were men (38.5%). As the median interval time from admission to MSC infusion in the MSC group was 18 days, 18±2 days after admission was selected as the baseline in the control group. Two patients were considered critical upon admission. Baseline characteristics and laboratory biomarker levels, including age, sex, WBC, Neu, Lym, BUN, Cr, CRP, IL-6, IL-10, and TNF-α, were comparable between the MSC-treated and control groups (p>0.05, Table 2).
Comparison of baseline characteristics between the MSC-therapy and control groups.
| Characteristics | Age | Gender | WBC | Neu | Lym | Hb | PLT | CRP | PCT | IL-6 | IL-1β | IL-2 receptor | IL-8 | IL-10 | TNF-α | ALT | AST | TB | BUN | Cr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p value* | 0.276 | 0.434 | 0.430 | 0.415 | 0.807 | 0.579 | 0.098 | 0.724 | 1.000 | 0.828 | 0.303 | 0.870 | 0.140 | 0.184 | 0.277 | 0.200 | 0.663 | 0.703 | 0.724 | 0.134 |
WBC, white blood cell count; Neu, neutrophil count; Lym, lymphocyte count; Hb, hemoglobin; CRP, C-reactive protein; PCT, procalcitonin; IL, interleukin; TNF, tumor necrosis factor; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TB, total bilirubin; BUN, blood urea nitrogen; Cr, creatinine. *Student's t-test or Mann-Whitney U test according to the characteristics of the distribution.
The median oxygenation index on admission was 321 (IQR: 170-455) mmHg. The oxygenation index of eight patients was also re-evaluated from days 12 to 16 after MSC therapy and was found to be significantly improved compared with the baseline value (median: 385 mmHg vs. 195 mmHg, p=0.012). The oxygenation index in four patients was not determined after MSC therapy because of the relatively mild state and evident improvement in the patients' conditions (the oxygenation indexes on admission for the four patients were 376, 467, 419, and 414 mmHg, respectively).
The levels of the biomarkers 1-3 days before MSC therapy, 5-8 days after MSC therapy, and 12-16 days after MSC therapy were analyzed. Although the levels of Lym tended to be higher and those of CRP, IL-6, and TNF-α tended to be lower, there was no significant difference in the levels of the biomarkers at the three time points (p>0.05, Table 3).
Comparisons of biomarker levels at three time points in patients treated with mesenchymal stem cell.
| Days 1 to 3 before MSC | Days 5 to 8 after MSC | Days 12 to 16 after MSC | p value* | |
|---|---|---|---|---|
| WBC (109/L)# | 5.85 (4.35, 8.59) | 6.85 (5.78, 8.88) | 6.13 (4.08, 7.31) | 0.531 |
| Neu (109/L)# | 3.37 (2.48, 4.91) | 4.38 (2.62, 6.32) | 3.78 (2.11, 4.64) | 0.644 |
| Lym (109/L)# | 1.27 (1.12, 1.82) | 1.69 (1.16, 1.88) | 1.50 (1.17, 2.05) | 0.582 |
| Hb (g/L) | 114.8±16.4 | 115.3±14.4 | 117.6±12.5 | 0.881 |
| PLT (109/L) | 213.6±67.0 | 182.8±68.0 | 183.7±59.1 | 0.436 |
| CRP (mg/L)# | 2.70 (0.93, 18.98) | 1.55 (1.23, 6.08) | 1.35 (0.63, 11.25) | 0.548 |
| PCT (ng/mL)# | 0.08 (0.06, 0.24) | 0.08 (0.06, 0.14) | 0.07 (0.06, 0.13) | 0.717 |
| IL-6 (pg/mL)# | 4.23 (2.98, 21.00) | 3.38 (2.27, 18.83) | 3.41 (2.84, 18.42) | 0.879 |
| IL-1β (pg/mL)# | 5.0 (5.0-5.0) | 5.0 (5.0-8.9) | 5.0 (5.0-25.9) | 0.359 |
| IL-2 receptor (U/mL)# | 623.5 (458.3, 843.0) | 543.0 (466.3, 590.3) | 489.0 (324.8, 583.8) | 0.345 |
| IL-8 (pg/mL)# | 13.9 (8.3, 28.5) | 15.5 (9.3, 19.8) | 17.8 (9.8, 23.4) | 0.730 |
| IL-10 (pg/mL)# | 5.0 (5.0, 5.2) | 5.0 (5.0, 5.6) | 5.0 (5.0, 6.3) | 0.938 |
| TNF-α (pg/mL)# | 12.3 (9.2, 18.7) | 11.2 (9.6, 13.2) | 10.1 (8.9, 12.1) | 0.580 |
| ALT (U/L)# | 20.5 (17.0, 28.8) | 22.5 (14.8, 40.8) | 24.0 (15.8, 35.0) | 0.789 |
| AST (U/L)# | 26.0 (20.3, 28.8) | 26.0 (15.3, 52.0) | 20.5 (14.3, 32.5) | 0.591 |
| TB (umol/L)# | 9.4 (8.3, 17.4) | 8.2 (7.1, 10.2) | 9.0 (6.6, 11.4) | 0.526 |
| BUN (mmol/L)# | 3.95 (3.25, 6.98) | 5.15 (4.33, 7.60) | 5.35 (4.63, 6.20) | 0.333 |
| Cr (umol/L)# | 67.5 (45.0, 89.0) | 61.0 (43.2, 99.5) | 65.0 (44.3, 75.5) | 0.867 |
WBC, white blood cell count; Neu, neutrophil count; Lym, lymphocyte count; Hb, hemoglobin; CRP, C-reactive protein; PCT, procalcitonin; IL, interleukin; TNF, tumor necrosis factor; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TB, total bilirubin; BUN, blood urea nitrogen; Cr, creatinine. #Median (interquartile range). *One-way analysis of variance or Kruskal-Wallis test according to the characteristics of the distribution.
The levels of IgM and IgG at days 5-8 and 12-16 after MSC therapy were also analyzed. Decreased levels of IgM were observed (median: 34.92 vs. 11.39, p=0.023), and the levels of IgG showed no significant change (median: 76.68 vs. 87.71, p=0.929).
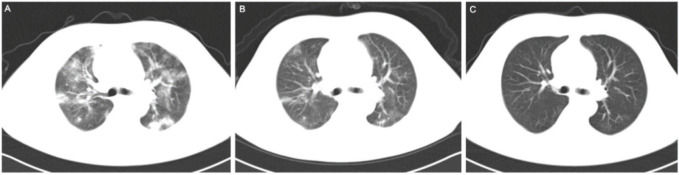
Pulmonary imaging in the treatment group improved to varying degrees, except that for patient 7. Figure 1 shows CT images of a patient with COVID-19 treated with MSCs. The CT images of 11 patients treated with MSCs were quantitatively evaluated. The area of pulmonary inflammation decreased significantly (median of 5888.3 mm2 before MSC treatment vs. median of 4108.0 mm2 after MSC treatment, p=0.003), and the CT number of the inflammatory area also tended to be restored after MSC therapy (median of -556.7 Hu before MSC treatment vs. median of -661.3 Hu after MSC treatment, p=0.062).
In the control group, the levels of the biomarkers at baseline as well as 5-8 days and 12-16 days after admission also showed no statistical difference (p>0.05, Table 4).
Comparisons of biomarker levels at three time points in the control group.
| Baseline | Days 5 to 8 | Days 12 to 16 | p value* | |
|---|---|---|---|---|
| WBC (109/L)# | 6.64 (4.86, 9.35) | 6.33 (5.94, 7.94) | 5.93 (5.02, 7.03) | 0.580 |
| Neu (109/L)# | 4.00 (2.76, 7.06) | 3.93 (3.48, 6.46) | 3.22 (2.83, 5.51) | 0.737 |
| Lym (109/L)# | 1.33 (0.80, 1.75) | 1.45 (0.73, 1.89) | 1.53 (0.76, 1.77) | 0.996 |
| Hb (g/L) | 119.0±20.9 | 111.3±20.5 | 111.4±20.4 | 0.555 |
| PLT (109/L) | 290.9±141.9 | 212.2±82.4 | 209.7±69.1 | 0.086 |
| CRP (mg/L)# | 4.30 (1.00, 24.35) | 4.20 (1.30, 54.30) | 2.30 (1.30, 65.20) | 0.886 |
| PCT (ng/mL)# | 0.08 (0.06, 0.29) | 0.07 (0.05, 0.26) | 0.07 (0.05, 0.31) | 0.856 |
| IL-6 (pg/mL)# | 5.76 (2.01, 37.96) | 6.09 (2.48, 44.62) | 4.07 (2.35, 41.49) | 0.970 |
| IL-1β (pg/mL)# | 5.0 (5.0-7.2) | 5.0 (5.0-6.9) | 5.0 (5.0-7.4) | 0.768 |
| IL-2 receptor (U/mL)# | 595.0 (386.5, 909.5) | 564.0 (359.0, 784.0) | 456.0 (348.5, 848.0) | 0.871 |
| IL-8 (pg/mL)# | 8.7 (5.0, 15.6) | 7.2 (5.4, 19.4) | 9.8 (5.2, 30.4) | 0.892 |
| IL-10 (pg/mL)# | 5.0 (5.0, 21.3) | 5.0 (5.0, 8.2) | 5.0 (5.0, 12.8) | 0.600 |
| TNF-α (pg/mL)# | 10.1 (6.1, 17.1) | 9.4 (6.0, 12.8) | 5.6 (8.1, 18.9) | 0.886 |
| ALT (U/L)# | 29.0 (18.5, 38.0) | 22.0 (11.0, 31.5) | 23.0 (12.0, 37.5) | 0.270 |
| AST (U/L)# | 28.0 (19.0, 37.5) | 20.0 (15.0, 23.0) | 20.0 (14.0, 34.5) | 0.264 |
| TB (umol/L)# | 9.8 (7.2, 15.7) | 9.2 (7.3, 13.1) | 8.6 (6.3, 13.8) | 0.609 |
| BUN (mmol/L)# | 3.15 (4.70, 9.60) | 5.00 (3.10, 8.30) | 4.70 (3.05, 9.20) | 0.972 |
| Cr (umol/L)# | 84.0 (58.0, 140.5) | 77.0 (52.0, 100.0) | 74.0 (49.0, 111.5) | 0.714 |
WBC, white blood cell count; Neu, neutrophil count; Lym, lymphocyte count; Hb, hemoglobin; CRP, C-reactive protein; PCT, procalcitonin; IL, interleukin; TNF, tumor necrosis factor; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TB, total bilirubin; BUN, blood urea nitrogen; Cr, creatinine #Median (interquartile range). *One-way analysis of variance or Kruskal-Wallis test according to the characteristics of the distribution.
Data on the levels of IgM and IgG at days 5-8 and 12-16 after admission at baseline were available for six patients in the control group. IgM and IgG levels revealed no significant changes (median: 102.38 vs. 52.65, p=0.249, and median: 85.17 vs. 93.06, p=0.600, respectively).
SafetyPatients in the treatment group were followed up for at least 60 days after MSC therapy. No allergic reactions were observed on the day of MSC infusion or thereafter.
Patient 7 from the MSC group died 17 days after MSC therapy due to respiratory failure, circulatory failure, and secondary infection. He was considered critical upon admission, and mechanical ventilation was used. MSC infusion was performed on day 23 after admission. The death was judged to be unrelated to MSC infusion by independent investigators. No other SAEs occurred in the MSC group throughout the observation period.
DISCUSSIONThe current study revealed an improvement in the oxygenation index and decreased levels of IgM in patients with COVID-19 treated with MSCs. Furthermore, pulmonary imaging in the MSC-treated patients also improved significantly. MSC infusion remains safe, with no observation of allergic reactions or the occurrence of infusion-related SAEs.
SARS-CoV-2 attacks the lungs and causes a series of pneumonia manifestations (3). MSCs have shown the ability to migrate into injured sites of the lung and to differentiate into functional alveolar cells; thus, further development of lung injury may be prevented, and the reconstruction of injured lung tissues is promoted (11). The aggregation of neutrophils and monocytes/macrophages may also be prevented because of the reduced permeability of the vascular wall induced by MSCs (12); therefore, inflammatory damage to the tissues and organs is mitigated. In the current study, the pulmonary imaging of patients treated with MSCs improved, the area of pulmonary inflammation decreased significantly, and the CT number of the inflammatory area also tended to be restored. Although previous studies have reported an improvement in chest CT imaging in patients after MSC transplantation (13), the quantitative measure for pulmonary imaging and relatively large sample size may have proven advantageous in the current study.
In addition to the improvement in morphological manifestations, lung function was restored. The current study also revealed that the oxygenation index in patients treated with MSCs was significantly improved compared with that at baseline, which is consistent with the results of previous studies (13,14). MSCs have been shown to inhibit the activities of dendritic cells, T lymphocytes, B lymphocytes, and natural killer cells; thus, the secretion of inflammatory cytokines, such as TNF-α, IL-1 β, and IL-6, decreases, while the levels of anti-inflammatory factors, such as IL-10, increase (4,11,12). Furthermore, immune dysfunction during the disease course may also be corrected (4,11,12). This improvement may be the main therapeutic mechanism of MSCs. However, patients in the control group of the current study were not evaluated for pulmonary imaging and oxygenation index; hence, the absence of a control may be the main limitation of the current study and that of previous studies (4,14).
Serological antibodies against SARS-CoV-2 have been used for diagnosis and prognosis prediction in clinical practice (15,16). A previous study indicated that an increase in IgG was positively correlated with a decrease in CRP level, which might have been associated with recovery from the disease (15,17). The exploration of antibody changes in patients with COVID-19 treated with MSCs is of interest because of their potential improvement in immunological function. However, few studies have focused on these indicators. The current study found a significant decrease in the level of IgM in patients treated with MSCs, while the levels of IgM and IgG were not significantly different in the control group. It is unclear whether a more rapid decrease in IgM predicts a better prognosis, and the relatively long-term changes in IgG levels should be further investigated in the future.
Cytokine storms have been shown to play important roles in the pathogenesis of COVID-19 (18). Previous studies have revealed the over-activation of CD4+ and CD8+ T lymphocytes, which are responsible for severe immune injury during the disease course (2,19). Higher levels of IL-6, IL-10, and TNFα have been observed in patients with severe or critical COVID-19 (2,). Gradually increasing cytokines were also detected during the course of the disease, especially in deteriorated patients (17,18,20). The ability of MSCs to relieve immune injury has been proven (4,11,12), and a decrease in inflammatory cytokines is expected. However, in the current study, no significant changes in cytokines were observed in either the MSC or control group. As we found no obvious cytokine storm when patients were treated with MSCs, the late application of MSCs may be responsible for the inconspicuous change. Combined with the results of previous studies (13,14,21), our findings suggest that the prognosis of COVID-19 may be enhanced when MSCs are used in the early stage of the inflammatory factor storm. Large-scale clinical studies are required to confirm this hypothesis.
The current study also evaluated the safety of MSC treatment and found no allergic reactions or transfusion-related SAEs, which was consistent with the findings of previous studies (13,14). Previous studies involving treatment with MSCs for diabetes and ankylosing spondylitis performed by our medical team also revealed favorable safety (22,23). The well-tolerated features of MSCs may be a sound basis for future studies.
The current study has certain limitations. First, as a pilot study, patients in the MSC-treated and control groups were not randomly recruited, and bias was ineluctable. Further randomized clinical studies that recruit more patients are required in the future. Second, there were missing data in this study. For example, the oxygenation index after MSC treatment was not assessed in four patients in the MSC-treated group. Third, given the severity of COVID-19, there is no alternative to the late application of MSCs. However, such a pilot study during the early outbreak phase in China provides valuable evidence that the treatment is moderately safe and efficacious. The use of the control group, quantitative measure for pulmonary imaging, and evaluation of antibodies strengthened the conclusions of the current study.
CONCLUSIONIn conclusion, intravenous transfusion of MSCs in patients with COVID-19 is effective and well tolerated. Further studies involving a large cohort with a proper clinical trial design and adherence to quality measures, including quality control of cell products, timing, and dosing regimens of treatments, are warranted.
AUTHOR CONTRIBUTIONSWei F, Kong D, Li T, Yu F, and Zheng C conceived and designed the study. Hong K, Cui Y, Tang S, and Tan Y performed the experiments. Wei F, Kong D, Li T, Li A, Fang J, Zhuang X, Lai C, Xu W, Dong H, and Ma C were responsible for the patient follow-up and data collection. Wei F, Kong D, Li T, Yu F, and Zheng C wrote and revised the manuscript, and analyzed the data.
We would like to thank the employees of the Yinfeng Biological Group and Shengsheng Supply Chain Management for the logistics and shipping of MSCs. We would like to thank Prof. A Drake for his critical reading of the manuscript. Prof. C Zheng is the recipient of the Rongxiang Regenerative Medicine Foundation of Shandong University 2019.