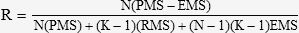Cold exposure induces glycogen and lipid depletion in the liver and the adrenal gland, respectively. However, no previous study has determined the effects of electrical countershock on those tissues. We aimed to evaluate the effects of electrical countershock on lipid depletion in the adrenal gland and on glycogen depletion in the liver.
METHODS:We used 40 male Wistar rats divided into four groups: the control group, in which the animals were subjected to a resting period of seven days; the electrical discharge group, in which the animals were subjected to a resting period followed by administration of ten 300-mV electrical discharges; the electrical post-discharge group, in which the animals received ten electrical shocks (300 mV) followed by rest for seven consecutive days; and the cold stress group, in which the animals were subjected to a resting period and were then exposed to −8ºC temperatures for four hours. All animals underwent a laparotomy after treatment. The lipid and glycogen depletions are presented using intensity levels (where + = low intensity and ++++ = high intensity, with intermediate levels in between).
RESULTS:The rats exposed to the cold stress presented the highest glycogen and lipid depletion in the liver and the adrenal gland, respectively. Furthermore, we noted that the electrical countershock significantly increased lipid depletion in the adrenal gland and glycogen depletion in the liver. One week after the electrical countershock, the liver and adrenal gland profiles were similar to that of the control group.
CONCLUSION:Electrical countershock immediately increased the glycogen depletion in the liver and the lipid depletion in the adrenal gland of rats.
More than 20 years ago, Selye1 recognized that the physiological system is activated by stress and that protecting and restoring the body can also damage the system.2,3 Exposure to low temperatures is considered to be an important stressing physical agent.4,5 Previous studies have reported that acute exposure to cold stress can damage heart tissue6 and cause lipid depletion in the adrenal gland and glycogen depletion in the liver,6–8 thus suggesting that sympathetic activity and oxidative stress are the sources of these injuries.
Previous studies have demonstrated that cardiomyocytes can be injured by electrical discharges. Under normal circumstances, clinical evaluation following defibrillation has revealed that the electrical energy pulse applied to the thoracic area has enough force to conserve the heart effectively, even though the discharge increases proportionally with patient body weight.9–12 Moreover, the magnitude of the damage depends on the electrical pulse waves, and thus, the myocardial injury is highlighted by the unevenness of the ST segment on the electrocardiogram. In addition, a minor aggression was reported when truncated biphasic shocks were applied instead of attenuated monophasic sinusoidal discharges.13
Although it was recently indicated that cold stress induces glycogen and lipid depletion in the liver and the adrenal gland, respectively,6,14 no previous study has investigated the effects of different types of stress on the liver and the adrenal gland. Furthermore, it was previously observed that electrical discharge in rats was able to produce cardiac injury noted through mitochondrial impairment in the heart tissue;9–11 therefore, we hypothesized that an electrical discharge would also affect other organs in addition to the heart. In this study, we aimed to evaluate the effects of two different types of stress agents on lipid depletion in the adrenal gland and on glycogen depletion in the liver.
METHODSAnimalsIn this study, experiments were performed on 40 adult male Wistar rats (250–350 g). The rats were obtained from the Central Biotery of our university. The temperature was maintained at 22ºC, air humidity was kept at nearly 60% and the 12 h light-12 h dark cycle was used. The animals had free access to food and water. After an adaptation period of nearly one week, the animals were randomly selected and separated into four groups: the control group (n=10), in which the animals were subjected to a resting period (seven days before experiments) with water and food ad libitum; the electrical discharge group (D, n=10), in which the animals were subjected to a resting period with water and food ad libitum followed by administration of ten 300-mV electrical discharges, in this group lipid and glycogen depletion were analyzed immediately after electrical discharge; the electrical post-discharge group (PD, n=10), in which the animals received ten electrical discharges (300 mV) and were then given water and food ad libitum for seven consecutive days, in this group lipid and glycogen depletion were analyzed seven days after electrical discharge; and the cold stress group (Cold, n=10), in which the animals were subjected to a resting period with water and food ad libitum and were then exposed to −8ºC for four hours only once. After the resting period laparotomy was performed in all animals, in order to evaluate lipid and glycogen depletion. Each group contained 10 rats because this number of animals provided sufficient statistical power while minimizing the number of animals used. All procedures were performed in accordance with the ethical guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the study protocol was approved by the Ethical Committee for research at our university.
Induced Hypothermia ProcedureFor the cold stress group, the rats were exposed to cold stress that was induced by placing them in wire mesh cages in an open refrigerated compartment at −8ºC for 4 hours. The rats were exposed to this environment only once, and their behavior was observed throughout the stress experiment.7 Measurements of the rats’ body temperatures confirmed that the rats were able to maintain a body temperature around 37C. The temperature was controlled by the refrigerated compartment. No rats died during the induced hypothermia procedure.6–8
Electrical CountershockElectrodes adapted for this study (3M®) were positioned on the animal’s precordial region to apply the electrical discharges. The electrical discharges were delivered through electrical cardioverter equipment adapted for small rodent animals by the bioengineering department of our university. The equipment was assembled to mimic the equipment used to treat cardiac rhythm reversion disorders in humans. The cardioverter device is able to generate serial voltage impulses of up to 300 mV, which corresponds to an energy transmission potential of 3 J. This serial voltage with the combined signal pattern is similar to a capacitor discharge in an RL circuit (inductance and resistance), with a 10 ms average. The impedance of the developed system for the experimental purpose was not determined due to the complexity and nature of the study methodology. However, the experimental group conditions were always equal, thus allowing a reliable and fair comparison.15
Histological ProcedureAll animals were submitted to a laparotomy after an adequate level of ether anesthesia was reached; adequate anesthesia was verified by tail tonus and response to external stimuli (i.e., through the evaluation of vibrissae movements) before and during the surgical procedure. Two pieces of the left lobe of the liver and the right adrenal gland were removed for investigation by light microscopy. The fragments were cut into small pieces of 1 mm3 and were post-fixed in a 1% OsO4 solution for 2 h; the samples were then dehydrated and embedded in araldite. The silver or gray thin sections (60–90 nm) were cut using a Porter-Blum MT-B ultramicrotome. The ultra-thin slices were mounted on copper silver grids with 200 patches and were stained with uranyl acetate and lead citrate. The glycogen depletion in the hepatocytes and the lipid depletion in the adrenal gland cells were evaluated by three different investigators. Data concerning lipid or glycogen depletion are presented using intensity levels (where + = low intensity and ++++ = high intensity, with levels in between). Each sample was examined by three independent investigators using the same standardized criteria.6–8
Statistical AnalysisTo evaluate the data associated with lipid depletion in the adrenal gland cells and with glycogen depletion in the hepatocytes, the Kruskal-Wallis and the Tukey post-hoc tests were applied to compare the independent groups. Agreement of the measurements from the three investigators was evaluated and analyzed using Bartko’s intraclass correlation coefficient (see equation below) according to the Fleiss guidelines16 (significance set at p < 0.05).
Bartko’s test formulaR = Bartko’s correlation index; PMS = Patients’ Mean Square; RMS = Researcher Mean Square; EMS = Error Mean Square; N = Number of events; K = Number of investigators.
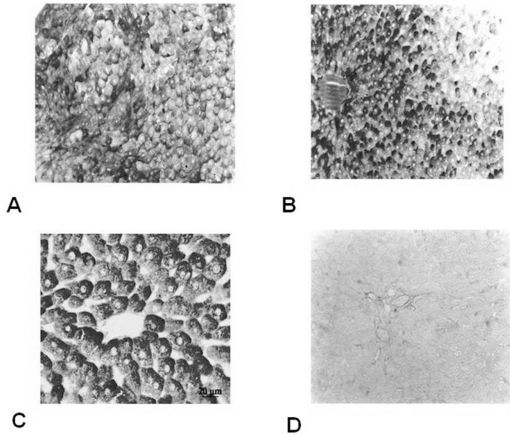
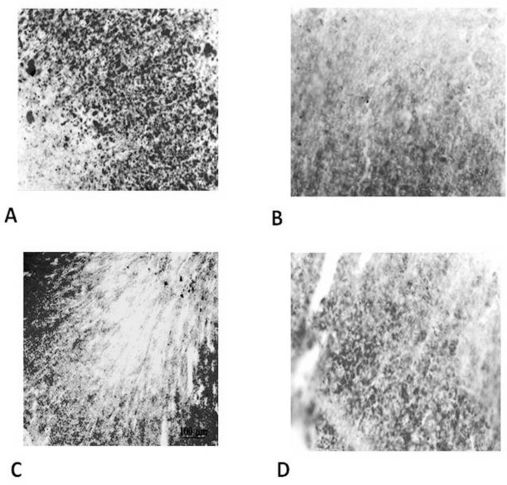
RESULTSWe observed that ten 300-mV electrical shocks altered the glycogen and lipid profiles in the liver (Figure 1 and Table 1) and the adrenal gland (Figure 2 and Table 2), respectively. However, one week after the electrical countershock, the liver and lipid characteristics were similar to that of the control group (Figures 1 and 2, Tables 1 and 2).
Glycogen depletion intensity in the hepatocytes stained with the PAS method. The range is from + = low intensity (higher glycogen depletion) to ++++ = high intensity (smaller glycogen depletion).
| Animal | Control | Discharge | Post-discharge | Cold |
|---|---|---|---|---|
| 1 | ++++ | ++ | ++++ | + |
| 2 | ++++ | +++ | ++++ | + |
| 3 | +++ | +++ | +++ | ++ |
| 4 | +++ | ++ | +++ | + |
| 5 | ++++ | ++ | +++ | ++ |
| 6 | +++ | ++ | ++++ | ++ |
| 7 | ++++ | ++ | ++++ | ++ |
| 8 | ++++ | ++ | +++ | + |
| 9 | +++ | ++ | ++++ | ++ |
| 10 | ++++ | ++ | ++++ | ++ |
Lipid depletion intensity in the adrenal gland cortical cells stained with Sudan IV. The range is from + = low intensity (higher lipid depletion) to ++++ = high intensity (smaller lipid depletion).
| Animal | Control | Discharge | Post-discharge | Stress |
|---|---|---|---|---|
| 1 | ++++ | ++ | ++++ | ++ |
| 2 | +++ | ++ | +++ | + |
| 3 | +++ | +++ | +++ | ++ |
| 4 | ++++ | ++ | +++ | ++ |
| 5 | ++++ | ++ | ++++ | ++ |
| 6 | ++++ | +++ | +++ | + |
| 7 | +++ | ++ | +++ | + |
| 8 | ++++ | ++ | ++ | ++ |
| 9 | ++++ | +++ | ++++ | + |
| 10 | ++++ | ++ | +++ | + |
As hypothesized, the induced hypothermia group presented the highest rate of glycogen depletion in the hepatocytes (Figure 1 and Table 1) (p<0.05) and the highest rate of lipid depletion in the adrenal gland cortical cells (Figure 2 and Table 2) (p<0.05). This result indicates that exposure to −8ºC temperatures for four hours was an effective stressing agent.
Bartko’s correlation index ranged between 0.56–0.95 in all experimental groups, thus validating the experimental methodology. Moreover, differences between the groups’ means were statistically significant (p<0.05).
DISCUSSIONIn this study, we investigated the effects of electrical countershock on lipid depletion and glycogen depletion in adrenal gland and liver cells, respectively, in Wistar rats. Because cold stress increases glycogen depletion in the liver and lipid depletion in the adrenal gland,6,8 we compared the intensities of the lipid and glycogen depletions between the cold exposure and the electrical shock groups. Moreover, to investigate if the liver and adrenal gland profiles change over time, we also evaluated the lipid and glycogen depletion one week after the electrical shock was applied. As hypothesized, the cold exposure increased the lipid depletion in the adrenal gland and the glycogen depletion in the hepatocytes, in agreement with previous studies.6,8 Furthermore, we noted that the electrical discharge immediately reduced the lipid and glycogen intensities in the adrenal gland and liver, respectively.
Behavioral analysis allowed us to observe the stress manifestation in the rats after exposure to −8ºC temperatures. Their behavior was similar to the initial reaction proposed by Selye1; moreover, Murad et al.17 observed similar reactions. In addition, the histological analysis of the liver tissue showed lower concentrations of glycogen in the hepatocytes of the rats exposed to cold stress. Stress conditions cause the release of catecholamine, which in turn causes oxidative glycogenolysis to occur; as a result, glycogenolysis is accelerated, leading to a lower concentration of glycogen in the cortical area of the liver cells.18 The histological analysis also demonstrated that the stress group presented a higher depletion of lipids in the cortical area of the adrenal gland cells. This is the result of the release of catecholamine, which stimulates lipolysis in the adrenal gland cells.18
According to our findings, electrical countershock immediately impaired the liver and adrenal gland profiles by causing further glycogen and lipid depletion, respectively. To the best of our knowledge, no study regarding this method of analysis has been previously published. As a result, we performed a morphometrical study of the changes in hepatocytes and adrenal gland cells caused by electrical countershock. A previous investigation found that electrical discharges in in vitro chicken cell cultures caused temporary microlesions in the cellular sarcolemma.19 Other experiments in dogs have confirmed the effects of direct current discharges on the mitochondria. Many mitochondrial pathological aspects, including loss of membrane integrity, swollen units and disruption, were found even after the application of low-energy endocardial countershocks.20,21 Considering the changes shown by electron microscopy on the seventh day in our procedures, two possibilities should be considered: constant effects based on the catecholaminergic action and/or the remission changes derived from the electrical discharges that were caused by the electrical discharge stress condition. A strong hypothesis relating other mechanisms to compensate for the physiologically unviable mitochondria, which were present mainly in the hepatocytes, must also be considered. The mitochondrial damage and the assumed reduction in ATP production may justify the glycogen depletion under the post-cardioversion and post-defibrillation conditions.20
The PD (post-electrical discharge) group was used to investigate the delayed liver and adrenal involvement caused by the adrenergic release. At seven days after the experimental procedures, we did not observe any hepatic glycogen or adrenal lipid depletion. Because of the animal adaptation period during the rest stage, the possibilities of dehydration, malnutrition, acid-base balance imbalances and metabolic disorders were eliminated. In addition, due to the short time that elapsed between the discharges and the collection of material for verification, systemic interference due to the catecholamine release is unlikely.
Our study shows that information concerning hepatic glycogen or adrenal lipid depletion should be considered in clinical myocardial protection, both before and after the clinical proceedings associated with transthoracic electrical discharges, particularly in subjects with heart disorders. However, the limitations of the present study were that we did not examine the systolic and diastolic blood pressure, heart rate, contractility, calcium homeostasis, or TnI levels. Additionally, we did not investigate other injury indicators, which could complement the countershock effects on the heart and cardiovascular parameters. We only studied the effects of the electrical discharges on the lipid and glycogen profiles in the adrenal glands and the liver, respectively, as this was the main focus of our research.
In conclusion, our findings showed that electrical countershock immediately depletes glycogen in the liver and lipids in the adrenal gland, similar to the effects of an acute cold stress. These effects were reversed one week after the electrical discharges were applied. Thus, these results show that the electrical discharge procedure induces liver and adrenal gland impairment.