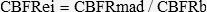We evaluated the impairment of endothelium-dependent and endothelium-independent coronary blood flow reserve after administration of intracoronary acetylcholine and adenosine, and its association with hypertensive cardiac disease.
INTRODUCTION:Coronary blood flow reserve reduction has been proposed as a mechanism for the progression of compensated left ventricular hypertrophy to ventricular dysfunction.
METHODS:Eighteen hypertensive patients with normal epicardial coronary arteries on angiography were divided into two groups according to left ventricular fractional shortening (FS). Group 1 (FS ≥0.25): n=8, FS=0.29 ± 0.03; Group 2 (FS <0.25): n=10, FS= 0.17 ± 0.03.
RESULTS:Baseline coronary blood flow was similar in both groups (Group 1: 80.15 ± 26.41 mL/min, Group 2: 100.09 ± 21.51 mL/min, p=NS). In response to adenosine, coronary blood flow increased to 265.1 ± 100.2 mL/min in Group 1 and to 300.8 ± 113.6 mL/min (p <0.05) in Group 2. Endothelium-independent coronary blood flow reserve was similar in both groups (Group 1: 3.31 ± 0.68 and Group 2: 2.97 ± 0.80, p=NS). In response to acetylcholine, coronary blood flow increased to 156.08 ± 36.79 mL/min in Group 1 and to 177.8 ± 83.6 mL/min in Group 2 (p <0.05). Endothelium-dependent coronary blood flow reserve was similar in the two groups (Group 1: 2.08 ± 0.74 and group Group 2: 1.76 ± 0.61, p=NS). Peak acetylcholine/peak adenosine coronary blood flow response (Group 1: 0.65 ± 0.27 and Group 2: 0.60 ± 0.17) and minimal coronary vascular resistance (Group 1: 0.48 ± 0.21 mmHg/mL/min and Group 2: 0.34 ± 0.12 mmHg/mL/min) were similar in both groups (p= NS). Casual diastolic blood pressure and end-systolic left ventricular stress were independently associated with FS.
CONCLUSIONS:In our hypertensive patients, endothelium-dependent and endothelium-independent coronary blood flow reserve vasodilator administrations had similar effects in patients with either normal or decreased left ventricular systolic function.
Left ventricular hypertrophy (LVH) in the context of systemic hypertension is an independent risk factor for sudden death, ventricular arrhythmia, myocardial ischemia, and heart failure.1 Many pathophysiological mechanisms, including reductions in coronary blood flow reserve (CBFR), have been proposed to explain the progression of compensated LVH to ventricular dysfunction.2 Coronary microcirculation alterations in hypertension have already been reported in a previous study.3 Antony et al. (1993) showed that CBFR was low in hypertensive patients both with and without LVH, but that following one year of antihypertensive treatment, reserve became normal in the non-LVH patients, suggesting that hypertension treatment may normalize compromised coronary blood flow.4
In patients with normal endothelium-independent CBFR, LV maladaptation as a result of inadequate flow may be explained by the occurrence of coronary vasculature endothelial dysfunction.3 This phenomenon has been described in the peripheral and coronary circulation of dilated cardiomyopathy patients.5 Endothelial dysfunction in hypertension has been shown in peripheral and coronary vasculature in patients without LV systolic dysfunction.3,6 It is possible that impairment of endothelium-dependent coronary vasodilatation capacity is one of the mechanisms responsible for inadequate myocardial flow, and therefore contributes to ventricular maladaptation before the development of morphological alterations that affect the capacity of endothelium-independent vasodilatation.
Given the multitude of factors involved in LV decompensation, no study to date has determined the roles of endothelium-dependent and independent coronary reserve compromise in different grades of hypertensive heart disease.
The goals of this study were: (1) to evaluate endothelium-dependent and independent coronary artery vasodilatation compromise in hypertensive patients with reduced or preserved fractional shortening; (2) to evaluate whether an association exists between LV dysfunction progression and endothelium-dependent and independent coronary reserve decrease; (3) to evaluate the influence of clinical, structural, and functional factors to LV maladaptation in hypertensive patients.
METHODSStudy population and groupsAt our institution, 33 hypertensive patients underwent clinical evaluation and additional routine laboratory examinations, 12-lead electrocardiography, chest X-ray, and transthoracic echocardiography. All patients were in NYHA functional class I or II, and the initial evaluation was performed using conventional antihypertensive medication.
Inclusion criteria were based on hypertension history, blood pressure (BP) levels above 140X90 mm Hg recorded on at least three occasions with the patient in a sitting position after resting for at least 5 minutes.7 Exclusion criteria were serum creatinine >2.0 mg/dL; presence of coronary artery stenosis >30%; contraindication for adenosine (previous chronic obstructive pulmonary disease, use of xanthine, bradyarrhythmia) or femoral access catheterization; iodate contrast allergy; use of acetylsalicylic acid, which could interfere with the behavior of coronary blood flow;8 and the presence of any other systemic or heart disease.
From the initial selected population, 12 patients were excluded due to significant coronary artery disease and three others were excluded due our inability to obtain an adequate coronary flow curve. Therefore, 18 patients were studied, divided into 2 groups based on left ventricular fractional shortening (LVFS). Group 1 exhibited preserved ventricular function and LVFS ≥25%, and Group 2 exhibited ventricular dysfunction and LVFS <25%. Group 1 comprised 8 patients (3 male, 50.88 ± 14.65 years). Group 2 comprised 10 patients (7 male, 48.7 ± 12.45 years). Baseline characteristics of the two groups are shown in Table 1. Patients from Group 1 were treated with hydrochlorothiazide (50 mg/day), methyldopa (750–1500 mg/day), amlodipine (10 mg/day), angiotensin conversion enzyme inhibitors (captopril 100–150 mg/day or enalapril 40 mg/day) or angiotensin receptor blocker (losartan 25–100 mg/day). Group 2 patients were treated with hydrochlorothiazide (50 mg/day), methyldopa (750–1500 mg/day), clonidine (0.2 mg/day), nifedipine (60 mg/day), angiotensin conversion enzyme inhibitors (captopril 150 mg/day or enalapril 10–40 mg/day), digoxin (0.25 mg/day), furosemide (40–80 mg/day), and spironolactone (25–100 mg/day).
Baseline characteristics of the two groups of patients
| Group 1 | Group 2 | P value | |
|---|---|---|---|
| Number of patients | n=8 | N=10 | |
| Age (years) | 50.88±14.65 | 48.7±12.45 | 0.7377 |
| Male | 3/8 (37.5%) | 7/10(70%) | 0.3416 |
| Race | 4 Caucasians (50%) | 3 Caucasians (30%) | |
| 4 Afro-americans (50%) | 6 Afro-americans (70%) | 0.3441 | |
| Body surface area (m2) | 1.68±0.13 | 1.60±0.20 | 0.3640 |
| Total cholesterol (mg/dL) | 191.63±27.00 | 184.80±19.94 | 0.5455 |
| LDL-cholesterol (mg/dL) | 123.63±17.74 | 114.00±25.18 | 0.3750 |
| HDL-cholesterol (mg/dL) | 48.75±12.87 | 46.20±15.65 | 0.7157 |
| Triglycerides (mg/dL) | 97.00±20.81 | 123.00±110.98 | 0.7223 |
| Glucose (mg/dL) | 99.00±6.76 | 96.10±12.07 | 0.5533 |
| Urea (mg/dL) | 31.25±7.59 | 40.60±8.72 | 0.0295 |
| Creatinine (mg/dL) | 0.94±0.14 | 1.13±0.23 | 0.0558 |
| Hemoglobin (g/dL) | 13.68±0.78 | 13.53±0.79 | 0.7023 |
| Hematocrit (%) | 40.25±1.49 | 40.60±2.55 | 0.4383 |
| Sodium (mEq/L) | 140.38±1.41 | 138.80±2.25 | 0.1043 |
| Potassium (mEq/L) | 4.04±0.49 | 4.25±0.31 | 0.2778 |
All patients had been diagnosed with hypertension at least 4 years prior to our study, and had been receiving initial antihypertensive treatment for at least 3.5 years.
Our hospital’s ethics committee approved this study protocol, # 696/00, and patients signed an informed consent form, in compliance with ethics committee guidelines.
Echocardiography studyTo determine LV structure and function, an echocardiographic study was performed, and the following parameters were determined according to American Society of Echocardiography recommendations:9 LV end-diastolic diameter (LVDD), LV end-systolic diameter (LVSD), interventricular septum (IVS), and posterior wall (PW) systolic and diastolic thickness. The equipment used was an ACUSON Sequoia 512 model (Acuson Corporation, Mountain View, California, EUA) equipped with a multifrequency transducer, model 3V2c.
We calculated LV mass corrected by body surface area (BSA) to obtain LV mass index (LVM).10 LVEF relative wall thickness (RWT).9 and LV end-systolic stress (LVFSS) were also studied.11
Hemodynamic studyPatients underwent right cardiac catheterization performed by the thermodilution method using a Swan-Ganz catheter, model 139f75 (Baxter Healthcare Corporation, Irvine, CA, USA) for pressure and cardiac output measurements.12 To measure LV aorta pressure, left cardiac catheterization was performed with cinecoronariography using non-ionic iopamidol iodated contrast (Iopamiron 370, Schering do Brasil, Química e Farmacêutica Ltda), and an intracoronary Doppler blood flow study. No left ventriculography was performed, in order to decrease the contrast dosage. Patients with significant coronary obstruction (>30%) did not undergo the coronary blood flow study.
Antihypertensive medication and vasodilative drugs were withheld for 24 to 48 hours before the study. Patients were advised to avoid eating and taking medications containing xanthines, due to the inhibiting effect these substances have on adenosine.
Coronary arterial flow studyFollowing the hemodynamic and cinecoronariography study, a dose of 10,000 UI heparin was administered by IV bolus. Afterward, a 6F or 7F (Boston Scientific Scimed, Inc., Maple Grove, MN, USA) catheter guide was positioned in the left coronary artery ostium. A 0.014-inch FloWire model guide wire (Cardiometrics, Inc., Mountain View, CA) with a FloMap model Doppler transducer (Cardiometrics, Inc., Mountain View, CA) on its extremity was introduced through the catheter and positioned in the proximal third of the anterior descending artery.
The following variables were evaluated: epicardial luminal cross-sectional area (CSA) at the location where flow velocity was measured, and coronary blood flow (CBF)13 Endothelium-independent hyperemia induction was performed with adenosine (Adenocard, Libbs Farmacêutica Ltda, Brasil), 18μg (AD1) and 36μg (AD2), by IV bolus3 Next, angiography was performed to determine cross-sectional diameter under these conditions to calculate CBF with AD1 and AD2. The greatest flow obtained (CBFRm ad) was used to calculate coronary blood flow reserve (CBFR), according to the formula:
where: CBFRe-i = coronary blood flow reserve endothelium-independent; CBFRm ad = maximum coronary blood flow reserve with adenosine; CBFRb = coronary blood flow reserve baseline.Acetylcholine was administered at a velocity of 5 mL/min for 2 minutes, with target concentrations of 10−7 (AC1), 10−6 (AC2), and 10−5 M (AC3) [14]. The endothelium-dependent CBFR was calculated according to the formula:
where: CBFRe i = endothelium-dependent coronary blood flow reserve; CBFRm ac= maximum coronary arterial flow reserve with acetylcholine; CBFRb= coronary blood flow reserve baseline.In order to evaluate whether infusion through acetylcholine dilution could increase flow due to shear stress, the acetylcholine study was preceded by a saline infusion at 5mL/min velocity for 2 minutes.6 To evaluate the extent of endothelium-dependent coronary vasodilation, the highest CBF obtained with acetylcholine (CBFRm ac) was divided by the highest CBF obtained with adenosine (CBFRm ad).3
Minimal coronary vascular resistance (RESISTmin) was calculated by evaluating the ratio between mean blood pressure (MBP ad) and maximum coronary blood flow using adenosine.3,8
At the end of the procedure, a 300 μg nitroglycerin dose (Tridil, Cristália Produtos Químicos Farmacêuticos Ltda, Brazil) was administered by IV bolus with the patient at baseline to evaluate endothelium-independent vasodilation capacity of the anterior descending coronary artery. Two minutes later, angiography was performed to determine the cross-sectional diameter under these conditions and the time necessary to achieve maximum epicardial artery dilation.3
Statistical analysisGroups 1 and 2 were compared using a Student t test or a Wilcoxon’s test for independent samples in relation to continuous variables evaluated at one time, in relation to RESISTmin and in relation to the adenosine and acetylcholine maximum flow ratio. For categorized variables, Groups 1 and 2 were compared using Fisher’s exact test.
Group comparisons relating to CBF, CFV, CF, SBP, DBP and MBP variables throughout baseline and adenosine 1 and 2 dose evaluations were performed using profile analysis. The same analyses were performed for group comparisons throughout baseline and acetylcholine 1, 2, and 3 dose evaluations, baseline and saline evaluations, and baseline and nitroglycerin evaluations. Occasionally, differences observed in hemodynamic variable behaviors (BP and heart rate) in response to vasodilating drugs (adenosine and acetylcholine) were considered in the endothelium-dependent and independent CFR calculation, using a covariance analysis.
The association of LVFS variables with SBP, DBP, MBP, age, LVM, RWT, LVFSS, RESISTmin, CBFRe i and CBFRe d variables (the latter 2 adjusted by covariance analysis) were evaluated using Pearson’s correlation coefficient and multiple linear regression. A stepwise method was adopted to identify variables with an entry significance level equal to 0.10 and an exit equal to 0.05. Data are expressed as means and standard deviations. p < 0.05 was considered significant.
RESULTSDemographic and laboratory variablesNo differences were found between groups in terms of age, sex, race, BSA, tabagism, or family history of coronary insufficiency. No difference was identified between groups based on laboratory test results, including total cholesterol level, LDL-cholesterol, HDL-cholesterol, or triglycerides, all of which were within normal ranges. Urea was statistically higher and creatinine tended to be higher in Group 2, but all values were still within the normal ranges (Table 1).
Left ventricle structure and functionThe LVDD (54.2 ± 5.9 mm x 69.0 ± 10.7 mm), LVSD (38.3 ± 4.4 mm x 57.4 ± 9.6 mm), LVM (201.5 ± 65.7 g/m2 x 310.4 ± 99.1 g/m2), and LVFSS (81.0 ± 19.9 103 dyn/cm2 x 123.99 ± 28.13 103 dyn/cm2) were lower in Group 1, and the RWT (0.44 ± 0.1 x 0.34 ± 0.1) was higher in Group 1 (p <0.05). No differences between IVS and PW thickness measurements were identified.
Hemodynamic studyAorta root blood pressures were higher in Group 1 compared to Group 2 [systolic (168.1 ± 28.5 mm Hg x 136.6 ± 31.0 mm Hg), diastolic (90.6 ± 11.5 mm Hg x 75.0 ± 12.3 mm Hg) and mean (117.4 ± 13.7 mm Hg x 95.9 ± 15.6 mm Hg)] (p <0.05). Cardiac index also tended to be higher in Group 1 (3.3 ± 0.6 L/min.m2 x 2.7 ± 0.5 L/min.m2) (p=0.0524). LV pulmonary capillary, central venous, and final diastolic pressures, pulmonary blood pressure (systolic, diastolic, and median), and systemic and pulmonary vascular resistance rates were not statistically different between groups.
Coronary blood flowIntracoronary drug administration was well tolerated. Saline solution infusion did not significantly change the anterior descending coronary artery diameter (Group 1, from 2.98 ± 0.58 to 3.00 ± 0.50 mm; Group 2, from 3.49 ± 0.34 to 3.54 ± 0.30 mm; p=0.2913), the coronary blood flow velocity (Group 1, from 23.88 ± 8.01 to 25.00 ± 8.77 cm/sec; Group 2, from 21.10 ± 4.98 to 22.30 ± 6.62 cm/sec; p=0.0850), heart rate (Group 1, from 73.88 ± 8.97 to 72.88 ± 10.22 bpm; Group 2, from 68.40 ± 9.77 to 67.70 ± 10.24 bpm; p=0.1712), SBP (Group 1, from 164.88 ± 25.39 to 161.50 ± 26.04 mmHg; Group 2, from 130.80 ± 26.81 to 131.40 ± 27.22 mmHg; p=0.2464) and MBP (Group 1, from 121.75 ± 14.87 to 118.13 ± 13.94 mmHg; Group 2, from 97.10 ± 14.41 to 97.70 ± 15.38 mmHg; p=0.1551). DBP decreased (Group 1, from 91.38 ± 10.98 to 88.88 ± 10.20mm Hg; Group 2, from 77.40 ± 11.47 to 75.10 ± 12.02 mm Hg; p=0.0176), comparably in both groups (p=0.9136).
Adenosine coronary blood flowAdenosine results are listed in Table 2. Profile analysis showed no differences in heart rate between the groups (p=0.2933) and no change in the two adenosine dose administrations (p=0.3139), in either group (p=0.2225). SBP and MBP were lower in Group 2 (p=0.0207 and p=0.0159, respectively), while DBP showed no difference between groups (p=0.0589). No SBP changes occurred in the two adenosine dose administrations at baseline (p=0.7207) in either group. DBP and MBP decreased (p=0.0005) comparably in both groups (p=0.6107 and p=0.1637 for DBP and MBP, respectively) for both adenosine doses in relation to baseline values. No difference in DBP (p=0.8332) or MBP (p=0.2008) were found from the two adenosine dose administrations in either group.
Hemodynamic variables, coronary blood flow velocity, and anterior descending coronary artery diameter with the use of adenosine
| Variables | Group 1 | Group 2 |
|---|---|---|
| HR-Basal (bpm | 73.88 ± 8.97 | 68.40 ± 9.77 |
| HR-AD1 (bpm) | 74.00 ± 8.54 | 69.20 ± 7.73 |
| HR-AD2 (bpm) | 73.75 ± 8.10 | 71.20 ± 8.32 |
| SBP-Basal (mm Hg) | 164.88 ± 25.39 | 130.80 ± 26.81* |
| SBP-AD1 (mm Hg) | 162.25 ± 29.74 | 130.10 ± 29.47* |
| SBP-AD2 (mm Hg) | 163.63 ± 26.73 | 129.40 ± 29.74* |
| DBP-Basal (mm Hg) | 91.38 ± 10.98 | 77.40 ± 11.47 |
| DBP-AD1 (mm Hg) | 80.13 ± 11.29# | 68.20 ± 14.85# |
| DBP-AD2 (mm Hg) | 79.25 ± 11.88# | 70.10 ± 17.15# |
| MBP-Basal (mm Hg) | 121.75 ± 14.87 | 97.10 ± 14.41* |
| MBP-AD1 (mm Hg) | 111.50 ± 17.08# | 93.40 ± 18.77*# |
| MBP-AD2 (mm Hg) | 107.25 ± 14.62# | 90.40 ± 18.51*# |
| CFV-Basal (cm/seg) | 23.88 ± 8.01 | 21.10 ± 4.98 |
| CFV-AD1 (cm/seg) | 62.88 ± 17.52# | 51.80 ± 17.89# |
| CFV-AD2 (cm/seg) | 65.25 ± 20.45# | 51.00 ± 17.42# |
| D-Basal (mm) | 2.98 ± 0.58 | 3.49 ± 0.34* |
| D-AD1 (mm) | 3.10 ± 0.60# | 3.67 ± 0.37*# |
| D-AD2 (mm) | 3.21 ± 0.64# | 3.74 ± 0.42*# |
AD1=18 μg adenosine; AD2=36 μg adenosine; HR=heart rate; SBP=systolic blood pressure; DBP=diastolic blood pressure; MBP=mean blood pressure; CFV=coronary flow velocity; D=anterior descending artery diameter;
*=p<0.05 vs. group 1;
#=p<0.05 vs. basal
Coronary blood flow velocity did not differ between groups (p=0.1715) and increased (p<0.0001) comparably in both groups (p=0.2716) in relation to baseline values (p<0.0001 for the two adenosine dose administrations). No difference was found in coronary blood flow velocity from the first to the second adenosine dose administrations in either group (p=0.6573, Figure 1).
Effect of intracoronary administration of adenosine (AD1=18 μg. AD2=36 μg) on coronary blood flow in Groups 1 and 2. Profile analysis showed that coronary blood flow did not differ between groups and increased comparably in relation to baseline values in both. No coronary blood flow difference from the first to the second adenosine dose administrations was evidenced in either group.
Anterior descending coronary artery diameter was greater in Group 2 than in Group 1 (p=0.0313). An increase (p=0.0005) in the anterior descending artery diameter was observed at the two adenosine dose administrations, which was comparable between groups (p=0.6605) compared with baseline values. No difference in the two adenosine dose administrations diameters occurred in either group (p=0.0734). Profile analysis showed that coronary blood flow did not differ between groups and increased comparably in relation to baseline values in both. No coronary blood flow difference from the first to the second adenosine dose administrations was evidenced in either group (Figure 1).
Acetylcholine coronary blood flowAcetylcholine results are listed in Table 3. Profile analysis showed that heart rate did not differ between groups (p>0.05) and changed differently in the two groups (p=0.0498). Heart rate at AC3 in relation to baseline values increased in Group 2 (p=0.0393), but decreased in Group 1 (p=0.0270). No difference in heart rate from AC1 to AC2 (p=0.08680) and from AC2 to AC3 (p=0.3101) occurred in Group 2, while in Group 1, heart rate decreased from AC1 to AC2 (p=0.0257), and no difference occurred from AC2 to AC3 (p=0.8835).
Hemodynamic variables, coronary blood flow velocity, and anterior descending coronary artery diameter with the use of acetylcholine
| Variables | GROUP 1 | GROUP 2 |
|---|---|---|
| HR-Basal (bpm | 73.88 ± 8.97 | 68.40 ± 9.77 |
| HR-AC1 (bpm) | 73.88 ± 8.11 | 68.70 ± 8.56 |
| HR-AC2 (bpm) | 71.13 ± 10.02α | 68.80 ± 8.50 |
| HR-AC3 (bpm) | 69.14 ± 8.25# | 69.22 ± 9.67# |
| SBP-Basal (mmHg) | 164.88 ± 25.39 | 130.80 ± 26.81* |
| SBP-AC1 (mmHg) | 166.75 ± 23.33 | 131.70 ± 31.13* |
| SBP-AC2 (mmHg) | 164.25 ± 26.52 | 126.90 ± 39.76* |
| SBP-AC3 (mmHg) | 166.00 ± 27.28 | 138.11 ± 28.09* |
| DBP-Basal (mmHg) | 91.38 ± 10.98 | 77.40 ± 11.47* |
| DBP-AC1 (mmHg) | 92.75 ± 12.00 | 77.20 ± 12.63* |
| DBP-AC2 (mmHg) | 89.38 ± 11.73 | 73.70 ± 22.31* |
| DBP-AC3 (mmHg) | 88.71 ± 9.23 | 80.22 ± 12.53* |
| MBP-Basal (mmHg) | 121.75 ± 14.87 | 97.10 ± 14.41* |
| MBP-AC1 (mmHg) | 124.50 ± 14.54 | 99.50 ± 17.33* |
| MBP-AC2 (mmHg) | 120.13 ± 14.72 | 95.40 ± 27.50* |
| MBP-AC3 (mmHg) | 119.00 ± 15.09 | 104.11 ± 14.90* |
| CFV-Basal (cm/seg) | 23.88 ± 8.01 | 21.10 ± 4.98 |
| CFV-AC1 (cm/seg) | 29.63 ± 13.61# | 24.80 ± 10.60# |
| CFV-AC2 (cm/seg) | 41.00 ± 19.87#α | 30.10 ± 14.97#α |
| CFV-AC3 (cm/seg) | 43.14 ± 25.40# | 34.67 ± 17.45# |
| D-Basal (mm) | 2.98 ± 0.58 | 3.49 ± 0.34 |
| D-AC1 (mm) | 3.06 ± 0.72# | 3.62 ± 0.40# |
| D-AC2 (mm) | 3.03 ± 0.67# | 3.60 ± 0.34# |
| D-AC3 (mm) | 3.06 ± 0.71τ | 3.26 ± 0.55τ |
AC1=10−7 M acetylcholine; AC2=10−6 M acetylcholine; AC3=10−5 M acetylcholine; HR= heart rate; SBP= systolic blood pressure; DBP= diastolic blood pressure; MBP= mean blood pressure; CFV= coronary flow velocity; D= anterior descending artery diameter;
*=p<0.05 vs. group 1;
# =p<0.05 vs. basal;
α=p<0.05 vs. AC1;
τ=p<0.05 vs. AC2
SBP, DBP, and MBP were lower in Group 2 (p=0.0425, p=0.0368, and p=0.0157, respectively). No differences in SBP, DBP, and MBP (p=0.8104, p=0.2526, and p=0.2455, respectively) occurred with the three acetylcholine dose administrations in relation to baseline values in either group.
Coronary blood flow velocity did not differ between groups (p=0.4706) and increased (p=0.0061) comparably (p=0.8837) in relation to baseline values in both (AC1, p=0.0167; AC2, p=0.0008; AC3, p=0.0011). An increase (p=0.0028) from AC1 to AC2 also occurred in both groups, and no coronary blood flow velocity difference was identified from AC2 to AC3 in any group (p=0.2583).
The anterior descending coronary diameter did not differ between the groups (p=0.1238) and increased (p=0.0289) comparably in both groups (p=0.6164) in relation to baseline values (AC1, p=0.0261; AC2, p=0.0434). AC3 diameter was comparable with baseline in both groups (p=0.1211). No diameter changes were noted from AC1 to AC2 (p=0.4530). A diameter decrease occurred from AC2 to AC3 (p=0.0073).
Two patients from Group 1 and four from Group 2 exhibited coronary artery spasms with the highest dose of acetylcholine (AC3). No analysis of these spasms was performed because they occurred in arterial segments that were not included in our measurements for the coronary blood flow calculation. No statistical differences between groups were identified in relation to the occurrence of these spasms.
Profile analysis showed that coronary blood flow did not differ between the groups but increased comparably in both in relation to baseline values. Coronary blood flow also increased between AC1 and AC2, but not from AC2 to AC3 in any group (Figure 2).
Effect of intracoronary administration of acetylcholine (AC1=10−7 M. AC2=10−6 M. AC3=10−5 M) on coronary blood flow in Groups 1 and 2. Profile analysis showed that coronary blood flow did not differ between the groups but increased comparably in both in relation to baseline values. Coronary blood flow also increased between AC1 and AC2, but not from AC2 to AC3 in any group.
The maximum coronary blood flow ratios using acetylcholine (CBFRm ac) and adenosine (CBFRm ad) were statistically comparable (p=0.6361) between Group 1 (0.65 ± 0.27) and Group 2 (0.60 ± 0.17).
Endothelium-independent coronary blood flow reserveAs SBP and DBP differed between the groups, endothelium-independent CBFR individual values were adjusted to baseline SBP and DBP values in the covariance analysis (COVAN). No endothelium-independent CBFR difference was seen between groups (p=0.4652).
Endothelium-dependent coronary blood flow reserveIndividual endothelium-dependent CBFR values were adjusted to baseline SBP and DBP values and were replaced by the difference between maximum and baseline CF in the covariance analysis (COVAN), as SBP and DBP differed between the groups and CBF was differently affected in each group. No endothelium-dependent CBFR difference occurred between groups (p=0.0772).
Minimal coronary blood resistanceRESISTmin did not differ between groups. The mean value for Group 1 was 0.48 ± 0.21 mm Hg/mL/min, and for Group 2 it was 0.34 ± 0.12 mm Hg/mL/min (p=0.0874).
Nitroglycerin epicardial anterior coronary artery vasodilatationThe anterior descending coronary artery diameter was larger (p=0.0253) in Group 2 (baseline=3.49 ± 0.34 mm; NG=3.91 ± 0.36 mm) than in Group 1 (baseline=2.9 8 ± 0.58 mm; NG=3.35 ± 0.60 mm) and increased (p=0.0002) comparably (p=0.8076) in both groups in relation to baseline values. Profile analysis showed that heart rate increased (p=0.0244), while SBP (p=0.0122), MBP (p=0.0035), and DBP (p=0.0118) decreased with nitroglycerin administration comparably in both groups.
LV fractional shortening and predictive factorsPearson’s correlation coefficient values with respective statistical probabilities of the simple linear regression analysis between LVFS and clinical study variables are shown in Table 4. Casual blood pressure measurements (SBP, DBP, MBP) were used in this analysis. A stepwise multiple linear regression analysis identified DBP and LVFSS as variables that were independently correlated to LVFS (adjusted r2 =0.7109; DBP p=0.0045; LVFSS: p=0.0002).
Pearson’s linear correlation between LVFS and study variables
| Variables | r | p |
|---|---|---|
| SBP | 0.51 | 0.0299 |
| DBP | 0.58 | 0.0114 |
| MBP | 0.57 | 0.0137 |
| Age | 0.01 | 0.9797 |
| LVM | −0.40 | 0.1037 |
| RWT | 0.68 | 0.0018 |
| LVFSS | −0.75 | 0.0004 |
| RESISTmin | 0.26 | 0.3007 |
| CBFRe i | 0.60 | 0.0137 |
| CBFRe d | 0.37 | 0.1257 |
SBP=systolic blood pressure; DBP=diastolic blood pressure; MBP=mean blood pressure; LVM= indexed left ventricular mass; RWT=relative wall thickness; LVFSS=LV end-systolic stress; CBFRe i=coronary blood flow reserve, endothelium independent; CBFRe d=coronary blood flow reserve, endothelium dependent.
Our study included hypertensive patients without other risk factors for heart disease (with the exception of one smoker). This study design allowed us to evaluate to the extent possible the independent effect of hypertension on CBFR. Although the study population consisted of patients with hypertensive heart disease who had been prescribed significant quantities of hypertensive medication, all were within NYHA functional classes I or II. In order to reduce the influence of hypertensive drugs on our results, all such medications were withheld for 24 to 48 hours before the study, as is common practice in the literature.3,5,6,8 It is widely accepted that one should wait 5 half-lives to eliminate the effects of a given drug - for some of the drugs used, 5 half-lives was longer than our chosen suspension duration. However, because our patients were at risk of hemodynamic decompensation, we did not consider it ethically acceptable to withhold their medications for an extended period.
Groups were assigned based on LV systolic function with statistically equivalent demographic and laboratory variables, and can therefore be considered comparable. However, echocardiographic evaluation confirmed a higher grade of LV remodeling and LV dysfunction in Group 2 compared to Group 1.
Hemodynamic characteristicsHemodynamic variables confirmed the good clinical compensation status of the patients, as filling pressures and cardiac index were at acceptable levels. We also note that no patients exhibited pulmonary artery hypertension. Groups did not differ in respect of ventricular filling pressures, a variable that could interfere with coronary blood flow measurements. The difference in LV afterload was evident because higher pressure levels were obtained at the aortic root in Group 1. The ultimate impact of CBFR in the context of this perfusion difference was not observed during the RESISTmin calculation, because no differences were identified between groups.
Coronary blood flow reserveWe chose to use nitroglycerin at the end of the study to evaluate whether the epicardial coronary artery response to an endothelium-independent stimulus was preserved. Therefore, in the event this arterial segment did not respond to flow induced by microcirculation dilation, we could conclude that it did not occur due to an inability of the vessel’s medium layer to respond to a vasodilatation stimulus, but rather due to a deficiency in the endothelium response to the increase in shear stress. Furthermore, as we observed, intracoronary nitroglycerin induces significant systemic hemodynamic alterations, such as a decrease in BP and an increase in heart rate, a fact that may interfere with CBF measurements. We performed isolated saline infusion, with a documented absence of measurable effects that supported the reliability of our results.
Our data suggest that endothelium-dependent and endothelium-independent CBFR vasodilator administrations were similar in patients with normal or decreased LV systolic function. Other studies have shown differences in endothelium-dependent and independent CBFR in cases of hypertension3,6 and dilated cardiomyopathy.5 In hypertension, the CBF dynamic is more dependent on structural than functional vascular alterations. It should be noted that the ratio between the maximum flow obtained with acetylcholine and that recorded with adenosine, an indication of endothelial function, was comparable in both study groups and was similar to normal values described in the literature.3,15 Additionally, the increase in epicardial diameter of the anterior descending coronary artery with adenosine was comparable between groups, reinforcing a similarity in endothelial function. Because adenosine acts primarily on vessel resistance,16 the induced diameter increase is understood to derive from high shear stresses consistent with an increased CBF, mediated by the endothelium.8,17 This occurs even when an epicardial artery diameter measurement taken when adenosine is used is recorded synchronously with a microcirculation action peak (15 to 20 seconds). The necessary waiting time for maximum flow-dependent vasodilatation response is 50 to 60 seconds, as demonstrated in humans.18 However, it is possible that a greater interval between adenosine administration and the documentation of epicardial coronary artery diameter could lead to different results.
The responses obtained with nitroglycerin show that conductance vessel vasodilatation capacity was comparable in both groups and was preserved, similar to previous reports.19 This normal vasodilatation response to nitroglycerin indicates a preserved epicardial coronary artery structure.
These results do not support the hypothesis that ischemic alterations caused by endothelial dysfunction explain LV functional maladaptation in hypertensive heart disease. Other studies have shown that hypertension, in the absence of other risk factors, is not associated with coronary endothelial dysfunction.3,15
Interestingly, although the two groups did not differ in endothelium-independent CBFR, there was a positive correlation with LVFS. In other words, the higher the ventricular dysfunction, the lower the reserve. However, this correlation was not evidenced in the multivariate analysis. The inverse correlation between LV systolic function and LVFSS was previously described in the context of hypertensive heart disease.20 In our study, LVFSS represented one of two variables independently related to LVFS. It is well known that one of the strongest coronary blood flow determinants is myocardial oxygen intake. Although CBFR does not correlate with LVFS in the multiple linear regression model given LVFSS and DBP variables, one must consider that, when facing an increase in systolic stress, the CBFR may be inadequate for the increased myocardial oxygen intake. Thus, if CBFR is not a determining factor for systolic dysfunction, its reduction may contribute to the continuation and/or aggravation of this clinical condition. This study shows that an association also exists between DBP and LV systolic function. The observed BP decrease in Group 2 can be explained in terms of deteriorating ventricular function.
It is important to emphasize that the study population consisted of hypertensive patients undergoing adequate long-term clinical treatment. It is possible that including patients who had not previously undergone treatment would have yielded different results. Antihypertensive drug treatment, particularly with calcium channel antagonists,21,22 angiotensin-converting enzyme inhibitors,23 and angiotensin-receptor blockers,24 can positively interfere with endothelial function and vascular remodeling. However, this fact does not diminish the significance of our findings. By contrast, it provides new perspectives for understanding how hypertension treatment can positively impact disease evolution.
Study limitationsIn this study, we did not use a normal control group, because this does not exist in practice, and it did not seem ethical to use an invasive methodology to conduct a characterization. In the literature, control groups consist of normal heart and angina patients with microcirculation disease (Syndrome X).25 We emphasize, however, that the values we obtained for endothelium-dependent and independent CBFR are very close to those reported in many studies.3,6,17
We thank Julia T. Fukushima for performing the statistical analysis.