In the ancient Indian system of medicine, Ayurveda, Bacopa monniera is classified as Medhya rasayana, which includes medicinal plants that rejuvenate intellect and memory. Here, we investigated the effect of a standardized extract of Bacopa monniera on the dendritic morphology of neurons in the basolateral amygdala, a region that is concerned with learning and memory.
METHODS:The present study was conducted on 2½-month-old Wistar rats. The rats were divided into 2-, 4- and 6-week treatment groups. Rats in each of these groups were further divided into 20 mg/kg, 40 mg/kg and 80 mg/kg dose groups (n = 8 for each dose). After the treatment period, treated rats and age-matched control rats were subjected to spatial learning (T-maze) and passive avoidance tests. Subsequently, these rats were killed by decapitation, the brains were removed, and the amygdaloid neurons were impregnated with silver nitrate (Golgi staining). Basolateral amygdaloid neurons were traced using camera lucida, and dendritic branching points (a measure of dendritic arborization) and dendritic intersections (a measure of dendritic length) were quantified. These data were compared with the data from the age-matched control rats.
RESULTS:The results showed an improvement in spatial learning performance and enhanced memory retention in rats treated with Bacopa monniera extract. Furthermore, a significant increase in dendritic length and the number of dendritic branching points was observed along the length of the dendrites of the basolateral amygdaloid neurons of rats treated with 40 mg/kg and 80 mg/kg of Bacopa monniera (BM) for longer periods of time (i.e., 4 and 6 weeks).
CONCLUSION:We conclude that constituents present in Bacopa monniera extract have neuronal dendritic growth-stimulating properties.
Bacopa monniera (BM), a traditional ayurvedic medicine, is reported to improve learning and memory behaviors in animals and humans.1-3 The plant and plant extracts have been extensively investigated for their neuropharmacological effects, and studies have confirmed their nootropic action.1-6 The active constituents of the plant facilitate learning and memory in normal rats and inhibit the amnesic effects of scopolamine, electroshock and immobilization stress.7,8 BM extracts have been reported to be non-toxic, non-teratogenic and non-mutagenic in rats and monkeys; single and multiple dosing studies in healthy human volunteers have not elicited adverse effects.9 In addition to its claimed memory-enhancing properties, BM has other potential benefits, such as a facilitatory effect on the capacity for mental retention.10,11 BM has been used in ayurvedic medicine and in traditional treatments for a number of disorders, particularly disorders that involve anxiety, intellect and poor memory.3 Significant antidepressant activity comparable to that of imipramine has been observed with the Brahmi extract after five days of oral administration, using a rodent model of depression.12 Additionally, anticholinesterase activity has been demonstrated.13
The amygdala is located deep within the medial temporal lobe, anterior to the hippocampus, close to the tail of the caudate nucleus. The amygdala is structurally diverse and comprises many nuclei. These nuclei are further divided into three major groups: 1) the deep or basolateral group; 2) the superficial or cortical-like group; and 3) the centromedial group.14 The amygdala is associated with a range of cognitive functions, including emotion, learning, memory, attention and perception.15 Most current views of amygdala function emphasize its role in negative emotions, such as fear, and in the linking of negative emotions with other aspects of cognition, such as learning and memory.16 However, recent studies of neuronal activity in the monkey amygdala have shown that, contrary to this widely held view, the amygdala is just as important for the processing of positive reward and reinforcement.17-19
In addition to its role in associative learning, the amygdala modulates memory processes that occur elsewhere, such as the hippocampus, the striatum18,21 and, perhaps, the prefrontal cortex. Many forms of emotionally driven memory tasks that are dependent on the amygdala and are impaired by pre-training amygdaloid lesions are spared by post-training amygdaloid lesions,22,23 which suggests that the amygdala is involved in only part of the learning process (i.e., adaptation). The role of the amygdala may not be limited to events that occur during training. Some studies have reported an increase or impairment of retention information when the amygdala is manipulated shortly after training, which suggests that the effects on memory are partly mediated by post-training neuronal activity.24,25
The hippocampus and amygdala interact with each other and also operate independently of each other, as shown by research on monkeys. In these monkeys, lesions of the hippocampus alone caused an impairment of the memory for object–place associations, but lesions of the amygdala alone caused an impairment of the memory for object-reward associations.26 The amygdala modulates both the encoding and the storage of hippocampal-dependent memories25 and is concerned with the programming of memories, especially those memories that are related to emotions.
Although there are reports that show that BM improves learning and memory in rats, there is no direct experimental evidence to show a correlation of the action of this plant extract on behavior, especially learning and memory, with histological changes in the brain. Our earlier study using BM extract treatment in adult rats showed improvements in learning and memory, including spatial learning and passive avoidance learning.34 Advanced neuroscience research has shown that the learning process is associated with alterations in the dendritic morphology of the hippocampal33-38 and amygdaloid neurons.33,39 The question of whether BM extract affects the neurons of the amygdala remains unanswered. Therefore, we attempted to answer whether this plant extract induced changes in amygdaloid neurons.
METHODSAnimals and experimental groupsWistar albino rats of random sex, approximately 2½ months old and weighing approximately 150 to 200 g were used for these studies. The animals were bred in the central animal house of Manipal University, Manipal, India. The rats were fed Amrut rat and mice pellets manufactured by Pranav Agro Industries Ltd, E/5-6, M.I.D.C., Kupwad Block, SANGLI – 416436 (Maharashtra), India. Four rats were housed in each polypropylene cage. All rats were maintained under a 12:12 hour cycle of darkness and light. The experimental protocol was approved by the Institutional Animal Ethical Committee for Experimental Clearance of Manipal University IAEC/KMC/02/2005-2006.
Rats were assigned to 2-, 4- and 6-week treatment groups. Rats in each of these groups were divided into 20 mg/kg, 40 mg/kg and 80 mg/kg dose groups (n = 8 for each dose). Each rat in a given dose group was fed the designated amount of standardized extract of BM daily for 2, 4 or 6 weeks. In addition to these experimental groups, an untreated normal control group (NC) and a gum acacia vehicle control group (GAC) (n = 8 in both groups) were also maintained.
Extraction and administration of BMStandardized plant extract of BM was supplied by the herbal manufacturer, M/s. Natural Remedies Private Limited, Bangalore, India. The shelf life of this extract is 2 years.
The first step was the extraction of the botanically identified plant material with alcohol. The alcoholic extract was re-extracted with water, and the water-soluble matter was retained. The final re-extraction was concentrated and dried to make a powder. Phytochemical analysis using high-performance liquid chromatography (HPLC) and high-performance thin layer chromatography (HPTLC) revealed that the final extract contained approximately 10% w/w (10% of the total mass of the extract) of the active ingredients (bacosides A and B).
The plant extract was administered orally with 5% gum acacia using an oral feeding needle attached to a syringe.
Behavioral testsFollowing BM treatment, all groups (NC, GAC and BM) were subjected to behavioral tests during the 12-hour dark period (which started at 7 PM). The behavioral tests consisted of a spatial learning (T-maze) test and a passive avoidance test and were performed as previously detailed.34
Rapid Golgi staining procedureAt the end of the treatment period (2, 4 or 6 weeks), the rats were anesthetized with ether and killed by cervical dislocation. The brains were removed and fixed in rapid Golgi fixative. Tissue was processed for rapid Golgi staining as previously detailed.36 Briefly, tissues were fixed for 5 days in Golgi fixative and impregnated with a 1.5% aqueous silver nitrate solution for 48 hours. Sledge microtome sections of 120-μm thickness were excised, dehydrated, cleared and mounted with Distrin plasticizer xylene mounting media.
Camera lucida tracingEight to 10 basolateral amygdaloid neurons from each rat were traced using camera lucida. The basolateral amygdala exhibits pyramidal-like neurons. These cells have pyriform or pyramidal somata that vary in size, depending on the nucleus. The dendrites arise from the cell body. Normally, the morphology of all of these cells is the same, and there is no variation between cells. Dendritic branching points (a measure of dendritic arborization), dendritic intersections (a measure of dendritic length) and dendritic processes that arose from the somata of the amygdaloid neurons were counted (Fig. 1). Neurons with a minimal overlap of dendrites, heavily impregnated with silver nitrate and without truncated dendrites were selected for tracing.
Quantification of dendritic branching points and dendritic intersectionsThe concentric circle method of Sholl (1956) was used for dendritic quantification.40 Concentric circles on a transparent sheet with a radial distance of 20 μm between circles were used for dendritic quantification (i.e., dendritic branching points and intersections). The sheet was placed on a neuron tracing so that the center of the cell body of the neuron coincided with the center of the concentric circles. The number of branching points between the two concentric circles, i.e., within each successive 20-μm concentric zone (ring), was counted. The dendritic intersection was the point where a dendrite intersected a given concentric circle (Fig. 1). The dendritic intersections at each concentric circle were counted. Both branching points and intersections were counted up to a radial distance of 100 μm from the center of the somata. The mean number of dendritic branching points in each concentric zone and the number of dendritic intersections at each concentric circle were calculated. The number of dendritic processes that arose from the somata of amygdaloid neurons was also counted.
Statistical analysisThe data were analyzed using an analysis of variance (ANOVA) followed by Bonferroni's test (post hoc) using GraphPad Prism, version 2.01.
RESULTSThe rats treated with all doses of BM showed an improvement in spatial learning performance and enhanced memory retention compared with normal control rats.34 Amygdaloid neuronal dendritic analysis in rats treated with all doses of BM (20, 40 and 80 mg/kg) for 2 weeks did not reveal altered dendritic arborization. However, treatment with BM 40 and 80 mg/kg for 4 weeks (Figs. 2 and 3; Tables 1 and 2) and with BM extract 20, 40 and 80 mg/kg for 6 weeks resulted in a significant increase in dendritic length (dendritic intersections) and the number of branching points (Figs. 4 and 5; Tables 3 and 4). In addition, treatment for 6 weeks with higher doses of BM extract (40 and 80 mg/kg) increased the number of dendritic processes that arose from the somata (Table 3). There was no difference in dendritic length or branching pattern between the control and gum acacia-treated rats, which suggested that the daily handling of the rats (i.e., handling stress and vehicle) itself did not alter the dendritic pattern. Because there were no significant differences in dendritic length and branching, only the comparisons between the control and experimental groups were subsequently detailed, and all of the figures ignore the vehicle control group data.
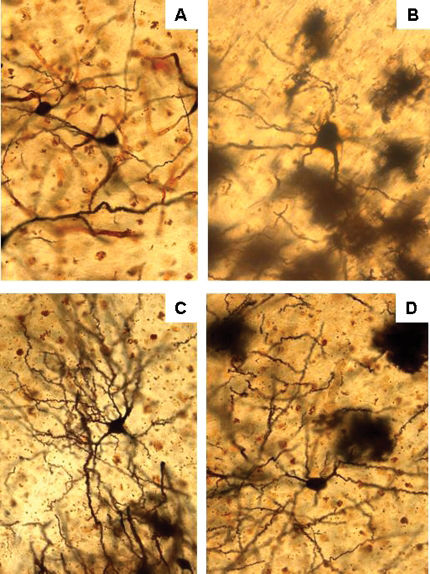
Photomicrographs of silver-nitrate-impregnated basolateral amygdaloid neurons from control rats A) and rats treated with Bacopa monniera, BM, for 4 weeks at doses of 20 mg/kg B), 40 mg/kg C) and 80 mg/kg D). A significant increase in dendritic arborization in the BM 40 and 80 mg/kg groups is demonstrated.
Basolateral amygdaloid neuronal dendritic intersections and the number of dendritic processes arising from the somata (4-week duration).
| Groups | n | Distance from soma, μm | Number of processes | ||||
|---|---|---|---|---|---|---|---|
| 20 | 40 | 60 | 80 | 100 | |||
| NC | 8 | 5.46 ± 1.23 | 6.61 ± 1.03 | 5.66 ± 1.21 | 4.07 ± 1.38 | 2.41 ± 0.69 | 4.43 ± 0.68 |
| GAC | 8 | 5.71 ± 0.82 | 6.47 ± 0.95 | 5.39 ± 1.32 | 3.94 ± 1.23 | 2.36 ± 0.54 | 4.27 ± 0.50 |
| BM 20 mg/kg | 8 | 5.68 ± 0.74 | 7.24 ± 0.83 | 6.87 ± 0.80 | 4.68 ± 0.76 | 3.02 ± 0.95 | 4.11 ± 0.64 |
| BM 40 mg/kg | 8 | 7.25 ± 0.59** | 10.32 ± 0.69*** | 7.98 ± 1.14** | 6.23 ± 0.90** | 2.98 ± 1.16 | 4.38 ± 0.71 |
| BM 80 mg/kg | 8 | 7.08 ± 0.76$$ | 9.86 ± 1.06$$$ | 7.69 ± 0.87$$ | 6.04 ± 0.82$$ | 3.14 ± 0.62 | 4.76 ± 0.45 |
BM, Bacopa monniera; GAC, gum acacia control; NC, normal control.
Note: Each value represents mean ± standard deviation. NC vs. BM 40 mg/kg: ** P<0.01, *** P<0.001; NC vs. BM 80 mg/kg: $$ P<0.01, $$$ P<0.001.
Basolateral amygdaloid neuronal dendritic branching points at different concentric zones and the total number of branching points (4-week duration).
| Groups | n | Concentric zones, μm | Total number of branching points | ||||
|---|---|---|---|---|---|---|---|
| 0-20 | 20-40 | 40-60 | 60-80 | 80-100 | |||
| NC | 8 | 1.32 ± 0.74 | 2.48 ± 0.57 | 1.08 ± 0.37 | 0.38 ± 0.25 | 0.15 ± 0.10 | 5.43 ± 1.67 |
| GAC | 8 | 1.23 ± 0.68 | 2.36 ± 0.63 | 0.97 ± 0.32 | 0.42 ± 0.30 | 0.12 ± 0.11 | 5.12 ± 1.54 |
| BM 20 mg/kg | 8 | 1.27 ± 0.53 | 2.72 ± 0.52 | 1.17 ± 0.50 | 0.56 ± 0.35 | 0.21 ± 0.13 | 6.08 ± 1.32 |
| BM 40 mg/kg | 8 | 2.32 ± 0.49 | 4.17 ± 0.75*** | 2.11 ± 0.72** | 1.07 ± 0.53* | 0.26 ± 0.22 | 10.03 ± 1.58*** |
| BM 80 mg/kg | 8 | 2.08 ± 0.64 | 4.03 ± 0.68$$$ | 2.06 ± 0.45$$ | 1.03 ± 0.46$ | 0.23 ± 0.18 | 9.46 ± 1.28$$$ |
BM, Bacopa monniera; GAC, gum acacia control; NC, normal control.
Note: Each value represents mean ± standard deviation. NC vs. BM 40 mg/kg: * P<0.05, ** P<0.01, *** P<0.001; NC vs. BM 80 mg/kg: $ P<0.05, $$ P<0.01, $$$ P<0.001.
Photomicrographs of silver-nitrate-impregnated basolateral amygdaloid neurons from control rats A) and rats treated with Bacopa monniera, BM, for 6 weeks at doses of 20 mg/kg B), 40 mg/kg C) and 80 mg/kg D). A significant increase in dendritic arborization in the BM 20-, 40- and 80-mg/kg groups is demonstrated.
Basolateral amygdaloid neuronal dendritic intersections and the number of dendritic processes arising from the somata (6-week duration).
| Groups | n | Distance from soma, μm | Number of processes | ||||
|---|---|---|---|---|---|---|---|
| 20 | 40 | 60 | 80 | 100 | |||
| NC | 8 | 5.34 ± 0.56 | 6.86 ± 1.12 | 6.39 ± 0.50 | 4.58 ± 0.71 | 3.45 ± 0.69 | 4.31 ± 0.64 |
| GAC | 8 | 5.87 ± 0.61 | 7.15 ± 0.75 | 6.25 ± 0.83 | 4.37 ± 0.68 | 3.34 ± 0.48 | 4.39 ± 0.58 |
| BM 20 mg/kg | 8 | 6.12 ± 0.65 | 9.08 ± 1.17## | 8.14 ± 1.25## | 5.18 ± 0.56 | 3.67 ± 0.44 | 4.85 ± 0.70 |
| BM 40 mg/kg | 8 | 7.60 ± 0.73*** | 12.18 ± 1.24*** | 11.81 ± 1.09*** | 8.13 ± 1.04*** | 5.08 ± 0.96** | 5.57 ± 0.62*** |
| BM 80 mg/kg | 8 | 7.38 ± 0.59$$$ | 11.69 ± 1.42$$$ | 11.47 ± 0.94$$$ | 7.79 ± 0.87$$$ | 4.93 ± 1.13$$ | 5.41 ± 0.43$$ |
BM, Bacopa monniera; GAC, gum acacia control; NC, normal control.
Note: Each value represents mean ± standard deviation. NC vs. BM 20 mg/kg: ## P<0.01; NC vs. BM 40 mg/kg: ** P<0.01, *** P<0.001; NC vs. BM 80 mg/kg: $$ P<0.01, $$$ P<0.001.
Basolateral amygdaloid neuronal dendritic branching points at different concentric zones and total number of branching points (6-week duration).
| Groups | n | Concentric zones, μm | Total number of branching points | ||||
|---|---|---|---|---|---|---|---|
| 0-20 | 20-40 | 40-60 | 60-80 | 80-100 | |||
| NC | 8 | 1.43 ± 0.45 | 2.68 ± 0.56 | 1.12 ± 0.32 | 0.52 ± 0.42 | 0.13 ± 0.10 | 5.91 ± 1.05 |
| GAC | 8 | 1.56 ± 0.42 | 2.76 ± 0.61 | 1.08 ± 0.43 | 0.48 ± 0.39 | 0.11 ± 0.09 | 6.01 ± 1.10 |
| BM 20 mg/kg | 8 | 1.65 ± 0.64 | 3.86 ± 0.67# | 1.93 ± 0.64# | 0.61 ± 0.41 | 0.23 ± 0.13 | 8.15 ± 0.98## |
| BM 40 mg/kg | 8 | 3.16 ± 0.70*** | 5.26 ± 0.75*** | 2.92 ± 0.55*** | 1.40 ± 0.62* | 0.46 ± 0.22* | 13.21 ± 1.20*** |
| BM 80 mg/kg | 8 | 2.92 ± 0.81$$$ | 5.08 ± 0.83$$$ | 2.85 ± 0.61$$$ | 1.28 ± 0.54$ | 0.42 ± 0.29$ | 12.55 ± 1.35$$$ |
BM, Bacopa monniera; GAC, gum acacia control; NC, normal control.
Note: Each value represents mean ± standard deviation. NC vs. BM 20 mg/kg: # P<0.05, ## P<0.01; NC vs. BM 40 mg/kg: * P<0.05, *** P<0.001; NC vs. BM 80 mg/kg: $ P<0.05, $$$ P<0.001.
There were no significant changes in dendritic intersections at any of the concentric circles in the BM 20-mg/kg group when compared with the normal control group. Both the BM 40- and 80-mg/kg groups showed significant increases in dendritic intersections at 20 μm, 40 μm, 60 μm and 80 μm concentric circles.
Dendritic branching points in different concentric zones (Table 2)No significant changes were observed in the dendritic branching points in any of the concentric zones in the BM 20-mg/kg group. However, both the BM 40- and 80-mg/kg groups showed a significant increase in dendritic branching points in concentric zone 20 to 40 μm, concentric zone 40 to 60 μm, and concentric zone 60 to 80 μm.
Total number of branching points (Table 2)There was no significant change in the total number of branching points in the BM 20-mg/kg group. However, the total number of branching points was increased significantly in the BM 40- and 80-mg/kg groups.
Number of dendritic processes (Table 1)There were no significant differences in the number of dendritic processes that arose from the somata in any of the groups that were treated with BM (20, 40 and 80 mg/kg).
Six-week treatment groupDendritic intersections (Table 3)All of the groups that were treated with BM showed significant increases in dendritic intersections in the 40-μm and 60-μm concentric circles. In addition, the BM 40- and 80-mg/kg groups showed significantly increased numbers of dendritic intersections in the 20-μm, 80-μm and 100-μm concentric circles.
Dendritic branching points in different concentric zones (Table 4)All of the groups that were treated with BM showed significant increases in dendritic branching points in the 20- to 40-μm and 40- to 60-μm concentric zones. In addition, the BM 40- and 80-mg/kg groups showed significantly increased numbers of dendritic branching points in the 0- to 20-μm, 60- to 80-μm and 80- to 100-μm concentric zones.
Total number of branching points (Table 4)The total number of branching points was increased significantly in all three groups that were treated with BM (20, 40 and 80 mg/kg) compared with the normal control group.
Number of dendritic processes (Table 3)There were no significant differences in the number of dendritic processes that arose from the somata in the group treated with BM 20 mg/kg. However, the BM 40- and 80-mg/kg groups showed significantly higher numbers of dendritic processes that arose from the somata.
DISCUSSIONMany studies have provided evidence for the involvement of the amygdala in different forms of memory storage and learning. Flood et al. (1995) stated that the amygdala is the most sensitive brain region for memory enhancement.41 The amygdala plays a crucial and preferential role in avoidance learning compared with spatial learning.42 The amygdala is involved in the formation of enhanced declarative memory for emotionally arousing events.43-45 According to Richter, Levin and Akirav (2000), the amygdala is involved in emotional responses and the formation of emotional memories.28 The amygdala is also involved in the formation of enhanced long-term memory.46,47 This view is supported by Tabert et al. (2001), who reported that the amygdala facilitates long-term but not short-term memory consolidation of emotionally significant material,48 and it is the cholinergic neurons that project to the amygdala that play an important role in memory acquisition.49
The term “passive avoidance” is usually employed to describe experiments in which an animal learns to avoid a noxious event by suppressing a particular behavior. The passive avoidance test is a behavioral test that assesses memory retention. Rats quickly learn to avoid noxious stimuli, which evoke a highly emotional learning process, by suppressing an innate natural behavior, such as staying in dark corners. The passive avoidance test is a useful method for the assessment of an impairment in learning and for the detection of both the ability to retain this learned knowledge and its recall.50
In our earlier study, we examined the effect of BM on behavioral changes using the same rats that were used in the present study. The rats that were treated with all of the doses of BM showed a significant improvement in passive avoidance memory retention.34 This increased retention in rats treated with BM compared with normal control rats indicated an increase in the ability to retain avoidance memories, which implied possible effects of BM on amygdaloid neurons.
The present study showed that the basolateral amygdaloid neuronal dendrites of rats treated with BM 40 and 80 mg/kg for 4 weeks and with 20, 40 and 80 mg/kg for 6 weeks resulted in significant increases in dendritic length and number of branching points. In addition, treatment with higher doses of BM extract (40 and 80 mg/kg) for 6 weeks increased the number of dendritic processes that arose from the somata. Increased dendritic length and branching points of basolateral amygdaloid neurons in rats that were treated with higher doses of BM for longer durations in the present study suggested that these doses of BM were sufficient to induce structural changes in these neurons. Naturally, these changes could have profound effects on behavior because of the additional dendrites on these neurons that are available for the formation of new synapses. It should be noted from the results that a significant number of additional dendrites were formed closer to the somata of neurons. This result suggests that these new synapses may result in a more rapid and effective conduction of impulses, which probably is one of the reasons for the enhanced learning and memory in these rats that has been reported previously.34
The learning process is associated with alterations in the dendritic morphology of amygdaloid neurons.33,39 In this study, the enhanced passive avoidance learning and memory in the rats treated with BM supported the functionality of the increased dendritic arborization in amygdaloid neurons of these rats.
Dendrites are the major determinants of how neurons integrate and process incoming information; therefore, they play a vital role in the functional properties of neuronal circuits. The functional organization of the various parts of the brain is dynamic and can change in response to experimental manipulations. Alterations in synaptic function, neuronal membrane properties and axonal trajectories are associated with these changes. The BM extract used in this study may play a role in these manipulations and lead to dendritic alterations.
The BM extract may stimulate the release of neuromodulators that alter the activity of neurotransmitters that are involved in learning and memory, which thereby contributes to enhanced learning and memory. Our earlier study showed that BM extract treatment in rats improved spatial learning performance and enhanced memory retention.34 Similar changes in the amygdala form the basis for improved memory retention.
CONCLUSIONIn conclusion, the results of our experiment suggested that BM extract treatment in rats with higher doses for longer periods induced structural changes in basolateral amygdaloid neurons that improved learning and memory.
The authors sincerely thank M/s. Natural Remedies Private Limited for supplying the BM extract and Manipal University (MU) for providing the experimental facilities to perform this work.