Cardiac syndrome X is characterized by angina-like chest pain, a positive stress test, and normal coronary arteries. A patient's mean platelet volume, which potentially reflects platelet function and activity, is associated with coronary atherosclerosis and endothelial dysfunction. The aim of the present study was to evaluate the mean platelet volumes of patients with cardiac syndrome X, those with coronary artery disease and normal subjects.
METHODS:Two hundred thirty-six subjects (76 patients with cardiac syndrome X, 78 patients with coronary artery disease, and 82 controls) were enrolled in the study. All of the subjects were evaluated with a detailed medical history, physical examination, and biochemical analyses. The mean platelet volumes were compared between the three groups.
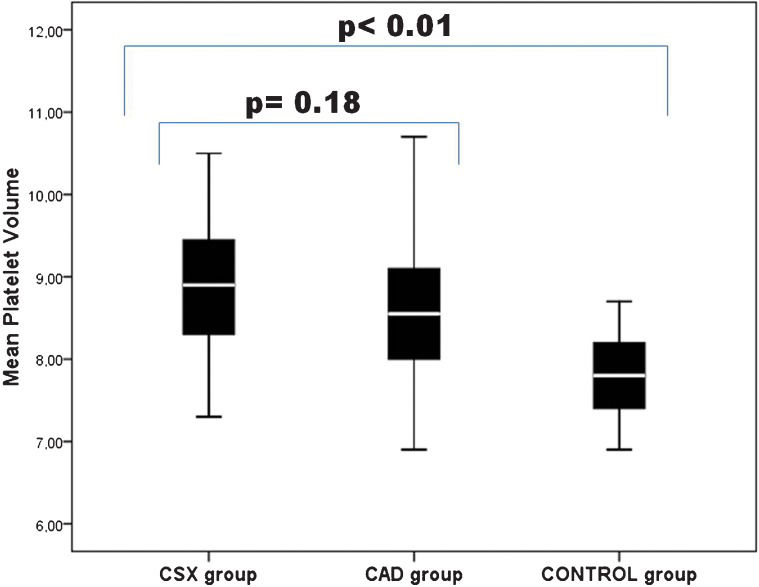
RESULTS:The mean platelet volumes in the patients with cardiac syndrome X and with coronary artery disease were significantly higher than those that were observed in the control group. There were no significant differences in the mean platelet volumes between the cardiac syndrome X and the coronary artery disease groups.
CONCLUSION:We have established that patients with cardiac syndrome X and coronary artery disease exhibit higher mean platelet volumes compared to controls. Patients with cardiac syndrome X exhibited higher mean platelet volumes compared to the controls, reflecting the presence of subclinical atherosclerosis. These findings suggest that, in addition to endothelial dysfunction, the presence of atherosclerosis may also contribute to the etiopathogenesis of cardiac syndrome X.
Cardiac syndrome X (CSX) is a clinical entity that must be distinguished from angina pectoris, the latter of which is due to typical obstructive coronary heart disease. CSX has the following three characteristic features: (i) angina or angina-like chest pain with exertion; (ii) ST segment depression that can be induced by treadmill exercise testing, or alternatively, a pathological thallium scan with normal coronary arteriography; and (iii) no spontaneous or inducible epicardial coronary artery spasm upon ergonovine or acetylcholine provocation (1). Several investigations have demonstrated that, despite normal coronary vessels, patients with CSX exhibit electrocardiographic as well as metabolic evidence of myocardial ischemia. Hence, the term “syndrome X” was first introduced by Kemp in 1973 to define this clinical entity (2). In addition, noncardiac causes of chest pain (e.g., esophageal disorders) or psychiatric conditions (e.g., panic disorder) should be excluded before CSX is diagnosed. Although the exact mechanism by which CSX develops, remains unclear, silent atherosclerosis and endothelial vasomotor dysfunction have been suggested as possible contributing factors.
The mean platelet volume (MPV) is an indicator of platelet activation, which is central to processes that are involved in coronary heart disease pathophysiology (3). The MPV is one of the platelet function indices that reflect the platelet production rate and platelet stimulation. Elevated MPVs are reported in cardiovascular diseases (4). However, as there are few data regarding the relationship between MPV and CSX, this study was designed to compare the MPVs of patients with CSX, patients with CAD, and of normal controls.
METHODSPatientsTwenty-eight hundred patients who had undergone coronary angiography between Jan 2010 and May 2011 in a tertiary referral center were retrospectively examined. Two hundred thirty-six patients were enrolled in the study and were divided into three groups. The CSX group consisted of 76 subjects (37 men and 39 women, mean age: 48.2±4.8 years). The diagnosis of CSX was based on the presence of a typical exercise-induced angina pectoris that was associated with either (i) transient ischemic ST segment depression (≥1 mm) during the treadmill exercise test (31 patients) or (ii) a reversible perfusion defect on myocardial perfusion scintigraphy (45 patients). Also required for a diagnosis were angiographically normal coronary arteries in the absence of coronary artery spasm (as determined by a hyperventilation maneuver). The CAD group consisted of 78 subjects (38 men and 40 women, mean age: 48.3±5.1 years) with CAD, which was defined as ≥50% stenosis in a minimum of one coronary artery. The control group consisted of 82 age- and sex-matched individuals (40 men and 42 women, mean age: 47.2±6.0 years) who presented with (i) anginal chest pain, (ii) a normal coronary angiography, and (iii) no ischemia on myocardial perfusion scintigraphy or during the treadmill exercise test. Written informed consent was obtained from all of the participants prior to enrollment. The Institutional Ethics Committee approved the study protocol, and the study was conducted in accordance with the Declaration of Helsinki. All of the subjects were evaluated with a detailed medical history, physical examination and biochemical analysis. Special emphasis was placed on cardiovascular risk factors and comorbid conditions. The blood samples were analyzed for levels of low-density lipoprotein cholesterol, high-density lipoprotein cholesterol and triglycerides (Beckman Coulter Syncron LX 20, Beckman Coulter, Brea, CA, USA). All of the subjects were questioned regarding cardiovascular drug use, smoking habits and alcohol consumption. All of the subjects underwent transthoracic echocardiography for structural heart disease. The image acquisition was performed when the subject was in the left lateral decubitus position. The images were obtained using a Vingmed System Vivid 7 (GE Vingmed Ultrasound, Horten, Norway), which was equipped with a standard two-dimensional transducer.
The exclusion criteria included the following: refusal to participate in the study and presence of hypertension, diabetes mellitus, left ventricular dysfunction (left ventricular ejection fraction <50%) or hypertrophy, unstable ischemic conditions (unstable angina pectoris and myocardial infarction), valvular heart disease, congenital heart disease, any observed abnormality in thyroid function tests, renal or hepatic dysfunction (creatinine >1.2 mg/dl, aspartate aminotransferase and alanine transaminase levels that were higher than double the upper limits of normal, respectively), inflammatory diseases, and any medication that could affect the MPV.
Cardiac catheterizationThe coronary angiograms were performed with a femoral approach using the Judkins technique, without the use of nitroglycerin, adenosine or calcium channel blockers. Following the appropriate patient preparation, all of the patients in the study population underwent elective coronary artery angiography using the Siemens Axiom Artis DFC (Siemens Medical Solutions, Erlangen, Germany). All of the angiograms were evaluated by two experienced physicians who were blinded to the study subjects' group assignments. The coronary angiograms were judged with regard to a smooth appearance, luminal wall irregularities, epicardial local or diffuse caliber reduction or stenosis. The coronary arteries were classified as normal on the basis of visual assessment of the absence of any luminal irregularities. To exclude the possibility of coronary artery vasospasm, the patients who exhibited normal coronary anatomy underwent a hyperventilation test during the coronary arteriography. The hyperventilation test was performed by asking the patients to breathe rapidly and deeply for 5 minutes.
Biochemical measurementsThe blood samples were withdrawn without stasis on the morning of the day prior to the coronary angiography and following a fasting period of 12 hours. The glucose, creatinine, and lipid profiles were determined using standard methods. For all of the groups, we measured the MPV from the blood samples that were obtained following the venipuncture. The blood was collected in tripotassium EDTA (7.2 mg) tubes. We analyzed the blood samples of all of the groups using an automatic blood counter after two hours of venipuncture. This period of time was waited to allow for the stabilization of platelet shape changes (Sysmex SE 9500, Roche).
Statistical analysesThe continuous variables are reported as the mean±standard deviation, whereas the categorical variables were defined as percentages. The data were tested for normal distributions using the Kolmogorov–Smirnov test. To compare the continuous variables, a one-way analysis of variance test or a Kruskal–Wallis test was used, as appropriate. When significant differences were observed between the three groups based on the post hoc analyses, either the Tukey or the Mann–Whitney U tests were used to determine the differences between the groups. A Chi-squared test was used to compare the categorical variables. Statistical significance was defined as p<0.05. The Statistical Package for the Social Sciences statistical software package version 15 for Windows (Statistical Package for the Social Sciences, Chicago, IL, USA) was used for the statistical analyses.
RESULTSThe primary characteristics of the study population are given in Table 1. There were no statistically significant differences between the three groups with respect to age, sex, smoking, or alcohol consumption (p>0.05). The body mass indexes, lipid profiles and fasting glucose levels did not differ between the three groups. Likewise, the laboratory characteristics of all of the groups were not significantly different (Table 1). There were no statistically significant differences in the MPV measurements between the CSX and the CAD groups (p =0.18) (Figure 1). The MPV was significantly higher in both the CSX and CAD groups compared with the control group (p<0.01) (Figure 1).
The baseline clinical and biochemical characteristics of the study groups.
| CSX group(n = 76) | CAD group(n = 78) | Control group(n = 82) | p-value | |
|---|---|---|---|---|
| Age, yrs | 48.2±4.8 | 48.3±5.1 | 47.2±6.0 | NS |
| Gender (M), n (%) | 37 (48) | 38 (48) | 40 (48) | NS |
| Active smokers, % | 26.1 | 24.5 | 27.2 | NS |
| Alcohol consumption, % | 12.1 | 10.8 | 9.82 | NS |
| BMI (kg/m2) | 26.9±6.5 | 25.1±5.8 | 7.2±6.0 | NS |
| Hemoglobin (g/dl) | 13.8±1.55 | 13.4±1.32 | 14.0±1.21 | NS |
| Fasting glucose (mg/dl) | 93±9 | 101±12 | 100±6 | NS |
| LDL cholesterol (mg/dl) | 119.0±28.1 | 113.0±33.3 | 114.7±22.9 | NS |
| HDL cholesterol (mg/dl) | 43.1±9.1 | 36.9±6.9 | 41.3±7.1 | NS |
| Triglycerides (mg/dl) | 148.6±56.8 | 155.6±71.9 | 142.8±58.8 | NS |
| Creatinine (mg/dl) | 1.02±0.13 | 0.97±0.22 | 0.96±0.17 | NS |
| Urea (mg/dl) | 32.8±5.0 | 34.9±8.8 | 33.1±8.2 | NS |
M: Male; BMI: Body mass index; LDL: Low density lipoprotein; HDL: High density lipoprotein; CSX: Coronary syndrome X; CAD: Coronary artery disease
The values are mean±SD.
In the present study, we demonstrate that the MPV was significantly increased in both CSX and CAD groups compared with controls. These results may indicate that elevated serum MPVs are associated with CSX-associated atherosclerosis.
The pathophysiology of CSX has not been clearly identified, although multiple abnormalities, including abnormal coronary flow reserve, insulin resistance, abnormal autonomic control, enhanced sodium hydrogen exchange activity, abnormal cardiac sensitivity, and microvascular spasm have been reported (5). All of these abnormalities may be associated with endothelial dysfunction. Therefore, it is unsurprising that the current hypotheses for the etiology of CSX focus on the vascular endothelium and the biological properties of arterial walls. The commonly accepted explanation for the role of the vasculature in CSX includes generalized endothelial dysfunction, inflammation, and increased pain sensitivity.
Platelets play an important role in the pathogenesis, morbidity, and mortality of acute coronary syndromes (6). Platelet function can be affected by several factors, such as platelet count, size, density, platelet age, a higher granule count, and adhesion receptor expression, as well as a higher mean platelet volume. The MPV appears to correlate more closely with platelet function than does platelet count alone (7). In previous reports, the following methods were used to analyze platelet activation: optical aggregometry, platelet function analysis using a PFA-100 system, platelet reactivity tests, platelet aggregate ratio measurements, flow cytometry and thromboxane B2 generation tests (8). All of these tests are of limited use due to complex preanalytic factors, reduced specificity and poor reproducibility. MPV is a simple marker that does not require advanced or expensive technology. However, the MPV may reflect platelet function and activity and may indirectly reflect platelet production and stimulation; therefore, elevated MPVs values may indicate cardiovascular diseases. Increased MPV may be due to the body's use of small platelets during acute ischemia (9). Thus, MPV has become a prognostic factor in coronary heart disease and may eventually be accepted as a parameter of platelet activity (10). In addition, several reports have demonstrated that there is a close relationship between MPV and cardiovascular risk factors, including impaired fasting glucose levels, diabetes mellitus, hypertension, hypercholesterolemia, obesity, and metabolic syndrome (11)–(13). It has been reported that elevated MPVs are associated with cardiovascular diseases (14). It is also known that increased platelet activation and aggregation are closely related to cardiovascular complications (10).
The origin of CSX is still debated; however, endothelial dysfunction, leading to a reduced coronary microvascular dilatory response and increased coronary resistance, are thought to play important roles. Abnormal subendocardial perfusion was detected using magnetic resonance imaging in patients with CSX (1). Cox et al. empirically demonstrated the presence of endothelial dysfunction and subangiographic atheroma in patients with CSX (15). Abnormal coronary arteries with atheromatous plaques and intimal thickening have been observed in intravascular ultrasonographic studies of patients with CSX (15,16). Therefore, the ethiopathogenesis of CSX may be similar to that of CAD. We therefore investigated the relationship between CSX and MPV and demonstrated a significant association between the two. In contrast to the present study, Cay et al. observed significantly higher MPVs in patients with CSX compared to those of patients with stable angina; however, the interpretation of this previous study is limited by its lack of a control group (17). In addition, the exclusion criteria were not as strict as those that were adopted in the present study. Furthermore, we excluded patients with hypertension, left ventricular dysfunction (left ventricular ejection fraction <50%) and hypertrophy, valvular heart disease, congenital heart disease, thyroid disease, and inflammatory diseases. Lastly, differences in the patient selection process may explain the discordant results between these two studies. The control group in the present study was selected based on complaints of anginal chest pain and a normal coronary angiography. Further inclusion criteria for the control group included the absence of ischemia both on myocardial perfusion scintigraphy and during the treadmill exercise test. Therefore, our study was designed to investigate the MPVs of patients with CSX or CAD compared with those of normal subjects.
The primary limitation of our study was the comparatively small size of the study population. Another limitation was the possibility of underlying coronary artery spasm in patients with CSX, which was excluded using a hyperventilation test, although the ergonovine test would have been the ideal test in this contest. Lastly, intravascular ultrasound (IVUS) provides more precise values than does coronary angiography and is more sensitive with respect to detecting and determining the distribution of coronary atherosclerotic plaques in the vessel lumen and throughout the wall. However, we were unable to perform IVUS in this study.
In conclusion, no statistically significant differences were observed with respect to MPV measurements between the CSX and the CAD groups. Relative to the controls, the MPV was significantly higher in patients with either CSX or CAD. These results suggest that elevated MPVs may participate in the pathogenesis of CSX. Prospective, placebo-controlled studies with large sample sizes that use multivariable survival analyses and long-term follow-up periods are required for the clinical evaluation of the prognostic value of increased MPV in patients with CSX.
No potential conflict of interest was reported.
Demirkol S contributed to the ideas for both the study and the manuscript, as well as to the data collection and manuscript writing. Balta S contributed to the data collection and the literature search. Unlu M contributed to both the manuscript preparation and data analysis. Yuksel UC contributed to the literature search and data collection. Celik T contributed to the critical review of the paper and data analysis. Arslan Z contributed to the statistical analyses. Kucuk U contributed to the writing and data collection. Yokusoglu M contributed to the oversight and organization.