Fibrin glues have not been consistently successful in preventing the dehiscence of high-risk colonic anastomoses. Fibrinogen and thrombin concentrations in glues determine their ability to function as sealants, healers, and/or adhesives. The objective of the current study was to compare the effects of different concentrations of fibrinogen and thrombin on bursting pressure, leaks, dehiscence, and morphology of high-risk ischemic colonic anastomoses using fibrin glue in rats.
METHODS:Colonic anastomoses in adult female Sprague-Dawley rats (weight, 250-350 g) treated with fibrin glue containing different concentrations of fibrinogen and thrombin were evaluated at post-operative day 5. The interventions were low-risk (normal) or high-risk (ischemic) end-to-end colonic anastomoses using polypropylene sutures and topical application of fibrinogen at high (120 mg/mL) or low (40 mg/mL) concentrations and thrombin at high (1000 IU/mL) or low (500 IU/mL) concentrations.
RESULTS:Ischemia alone, anastomosis alone, or both together reduced the bursting pressure. Glues containing a low fibrinogen concentration improved this parameter in all cases. High thrombin in combination with low fibrinogen also improved adherence exclusively in low-risk anastomoses. No differences were detected with respect to macroscopic parameters, histopathology, or hydroxyproline content at 5 days post-anastomosis.
CONCLUSIONS:Fibrin glue with a low fibrinogen content normalizes the bursting pressure of high-risk ischemic left-colon anastomoses in rats at day 5 after surgery.
Distal colonic anastomoses are exposed to the increased levels of bacteria and elevated intraluminal pressure characteristic of this organ. Thus, dehiscence is the main complication in colon surgery and is associated with high morbidity and mortality (1). Advanced age, malnutrition, malignancy, chemotherapy, immunosuppressive drugs, jaundice, uremia, diabetes, high-dose steroids, intra-abdominal infection, ischemia, irradiation, inadequate suture material, and a deficient surgical technique are factors that may contribute to anastomotic leaks, which are not always visible (2-4). Any of these conditions can increase the probability of microleaks, abscesses, or dehiscence by up to 80% (5). Thus, the implementation of techniques to protect these high-risk anastomoses is challenging. Synthetic adhesives, such as cyanoacrylate (6-8), and biological adhesives have been evaluated using different models of intestinal or colonic anastomosis (9); however, none of these adhesives have yielded good success rates. Carbohydrate polymers containing Zn have been tested experimentally, albeit only in duodenal perforation (10). The performance of fibrin glues has been consistently superior to that of other glues in experimental colonic low–risk anastomosis (11).
Fibrin glues, also called fibrin sealants, are biological adhesives derived from blood that depend on the final stages of the coagulation cascade (12) and were designed according to the mechanisms involved in this cascade; thus, fibrinogen is the main structural component in commercial products. The normal plasma concentration of fibrinogen is 2-5 mg/mL; however, it is present at a higher concentration in fibrin glues (120 mg/mL). Additionally, fibrin glues contain human or bovine thrombin at a concentration that varies from 400-1200 IU/mL, depending on the desired time for induction of a stable clot. Other components may include Ca2+ and tranexamic acid (92 mg/mL) or aprotinin; these compounds are antifibrinolytics that prevent the degradation of fibrin in the sealant (12-13).
Fibrin glues are advantageous because they distribute forces more evenly and less invasively than sutures or staples, are strong and flexible (11), and have been reported to reduce macro- and microhemorrhage, as well as exudate production, in intestinal anastomotic lines and increase hydroxyproline, an indicator of increased collagen production (14).
Fibrin glues have also been successfully used to increase the bursting pressure after anastomosis in the normal colon (15). Nevertheless, several experimental studies examining the use of fibrin glues in high-risk colonic anastomoses induced by radiation, peritonitis, ischemia, steroid therapy, or chemotherapy failed to demonstrate that this agent has positive effects on bursting pressure or perianastomotic infection (16-18). All these glues included fibrinogen at concentrations ranging from 120-1100 mg/mL; which is in contrast with other studies, where concentrations <120 mg/mL were associated with good results in the bowel as well as in low-risk colon anastomoses (19-21). High fibrinogen concentrations lead to enhanced hemostatic effects, whereas low concentrations allow for better adherence (22-23). Conversely, it has been reported that the adhesive strength of the glues increases with increasing thrombin concentration up to an optimal concentration of 50 IU/mL (24). These studies show that the concentrations of the components of fibrin glues may be critical for their application in different settings; however, to our knowledge, no study has been published comparing the effects of different concentrations of fibrinogen and thrombin, particularly in high-risk colonic anastomosis.
We designed this study to compare the effects of different concentrations of fibrinogen and thrombin on the adhesive force, leaks, dehiscence, and morphology of high-risk colonic anastomoses using fibrin glue in rats.
MATERIALS AND METHODSA total of 180 adult female Sprague-Dawley rats (weight, 250–350 g) were used. The animals were anesthetized with sodium pentobarbital (10 mg/kg, i.p.). Infraumbilical median laparotomy was subsequently performed, and the left colon was sectioned 3 cm from the peritoneal reflection. An end-to-end anastomosis was performed using 12 interrupted 7-0 polypropylene sutures. In the corresponding groups, a high-risk anastomosis was created via ligation of the mesenteric veins and arteries irrigating a 2 cm segment of the colon, with the anastomotic site in the middle. This cecal portion is also considered to be at high risk for anastomotic leakage (25). At this point, the animals were randomly assigned to different experimental groups according to fibrinogen and thrombin concentrations and to normal or high-risk anastomosis groups as shown in Table1. The rats were assigned to one of eight groups containing 20 rats each (n = 10 for bursting pressure measurements and n = 10 for histopathological assessment), and a control group was composed of 20 rats with intact colons (no anastomosis; n = 10) and devascularized colons (no anastomosis; n = 10) at one cm above and one cm below the point elected for anastomosis, with the aim of measuring the bursting pressure. Postoperative day 5 was chosen by our group because previous experiments with a similar model failed to demonstrate any resistance to bursting pressure during postoperative days 1-4.
Group assignment of rats according to colon status (normal or ischemic) and fibrinogen or thrombin concentration.
| Surgical condition | Risk | Group | N = 180 |
|---|---|---|---|
| Anastomosis without glue | NormalIschemic | NNNI | 2020 |
| High fibrinogen/high thrombin anastomosis | NormalIschemic | HNHI | 2020 |
| Low fibrinogen/high thrombin anastomosis | NormalIschemic | MNMI | 2020 |
| Low fibrinogen/low thrombin anastomosis | NormalIschemic | LNLI | 2020 |
| Intact colon | NormalIschemic | CNCI | 1010 |
High fibrinogen = 120 mg/mL; low fibrinogen = 40 mg/mL; high thrombin = 1000 IU/mL; low thrombin = 500 IU/mL.
Fibrin glue (Quixil, Omrix Biopharmaceuticals Ltd., Tel-Hashomer, Israel) was applied directly to the anastomosis just after it was completed. Dilutions of the fibrin glue components were prepared using injectable water. A total volume of 0.4 mL of sealant was applied to each anastomosis.
Bursting pressure measurementsFive days after surgery, rats in the bursting pressure groups were reanesthetized, and the laparotomy was reopened. The left colon was dissected, and a no. 18 venous catheter (Punzocath, Mexico) was inserted proximally. A 2-0 silk ligature was applied 2 cm proximal (over the catheter) and 2 cm distal to the anastomosis. A stopcock was then connected to the venous catheter, an infusion pump (Abbott Lifecare 2, IL), and a sphygmomanometer, which was zeroed to the level of the colon. A saline infusion was started at 1 mL/min, and the pressure was measured. A sudden decrease in pressure was indicative of colon rupture, and the maximal pressure reached was defined as the bursting pressure.
Histopathological assessmentFive days after surgery, rats in the histopathology groups were reanesthetized, and the laparotomy was reopened. The colon was resected 1 cm from either side of the anastomosis, and this ring was opened with a longitudinal incision and submerged in 10% phosphate-buffered saline (PBS)/formaldehyde, where it was maintained for later processing and blinded observation by two pathologists after staining with hematoxylin–eosin (HE), Masson's trichrome, or HE–alcian blue. Scores were assigned for histiocyte content assessment, ranging from 0 (incipient) to 1 (mild), 2 (moderate), and 3 (high histiocyte content). Fibroblast organization was classified as negative (0), moderate (1), or severe (2). Vascularization was assessed as vessels per field at a magnification of 200× and was graded as 1 (2–4 vessels per field), 2 (5–7 vessels per field), or 3 (8 or more vessels per field). All individual scores were added within each group to obtain a final number for analysis.
Hydroxyproline measurementsFragments of colon including the anastomosis and 2 cm on either side were hydrolyzed in 6 N HCl at 120°C for 12 h, and the hydrolysates were filtered and evaporated until dry. The remaining material was resuspended in distilled water and neutralized to pH 7.0. Hydroxyproline was quantified as described by Woessner (26). Considering that hydroxyproline constitutes 13.47% of the total amino acids present in the molecule, the collagen content was calculated as follows: collagen (mg) = 7.42× hydroxyproline (mg). The values are expressed as mg collagen/mg dry tissue.
Statistical analysisThe values are shown as the mean ± standard error of the mean unless specified otherwise. Comparisons were made between and among low- or high-risk groups using one-way analysis of variance (ANOVA) and post-hoc Student–Newman–Keuls tests. Regarding collagen content, the data from all groups failed normality testing based on the Kolmogorov–Smirnov test; thus, Kruskal-Wallis one-way ANOVA on ranks tests were performed. Histopathological grades were compared using a chi-square test. The significance was set at p<0.05.
EthicsThe study protocol was approved by the local animal research committee (CICUAL code 1999-0302009), and all animals were treated in compliance with institutional guidelines and Mexican federal regulations (NOM-062-ZOO-1999).
RESULTSMacroscopic and microscopic assessmentsOnly minimal macroscopic changes were observed. No significant differences were detected between groups with respect to abscesses, dehiscence, leaks, or acute microscopic peritonitis (data not shown). Histological changes were not observed (Figure1).
Collagen contentCollagen content, which was assessed based on hydroxyproline measurement, was not significantly different between the groups (Table2.
Colon hydroxyproline content in rats from all experimental groups. No significant differences were detected between or among groups (Kruskal–Wallis one-way ANOVA on ranks). The data are presented as the median/25–75% confidence interval.
| No glue | High fibrinogen/high thrombin | Low fibrinogen/high thrombin | Low fibrinogen/low thrombin | |
|---|---|---|---|---|
| No ischemia | 0.06/0.04–0.10 | 0.06/0.05–0.74 | 0.08/0.04–0.59 | 0.05/0.04–0.07 |
| Ischemia | 0.16/0.06–0.47 | 0.07/0.05–0.56 | 0.05/0.03–0.07 | 0.06/0.04–0.10 |
The high-risk model had a dramatic effect on outcome, as ischemia alone (without anastomosis) reduced the bursting pressure to 191±8 mmHg (group CI), compared with 323±25 mmHg in the normal non-sectioned colon (group CN) (Figures2 and 3). In addition, comparisons between normal and corresponding ischemic groups revealed significantly lower bursting pressure values in the presence of ischemia (p<0.01 for all groups, with the exception of HN vs. HI, which showed no difference).
The bursting pressure values in low-risk, non-ischemic anastomoses are shown in Figure2. As expected, all anastomoses reduced the bursting pressure compared with that of the untouched colon, with the exception of cases in which a low fibrinogen/high thrombin glue was used (MN). The latter group also exhibited improved bursting pressure compared with all other low-risk groups.
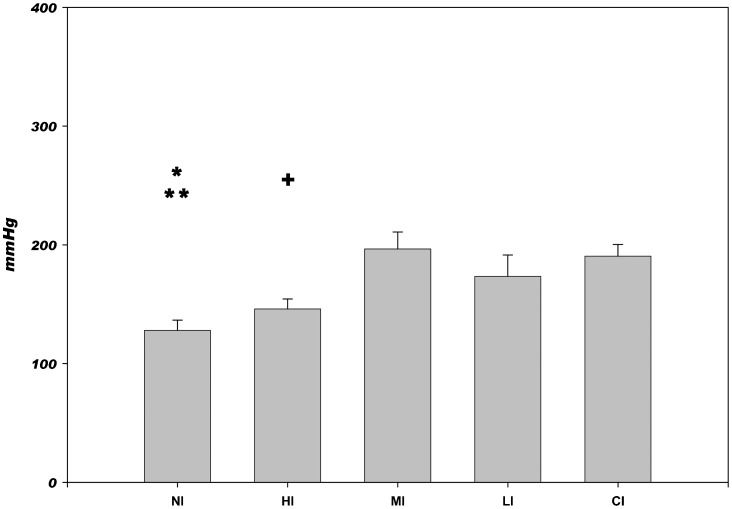
The bursting pressure values in high-risk ischemic anastomoses are shown in Figure3. The use of low levels of fibrinogen, either with high (MI) or low (LI) thrombin levels, improved the bursting pressure to the level of an ischemic untouched colon (CI). High levels of fibrinogen and thrombin in combination (HI) had no effect on this variable, and there was no difference observed compared with non-treated ischemic anastomoses (NI).
DISCUSSIONThe most relevant findings of this study are that ischemia and anastomosis alone or in combination significantly reduced the colon bursting pressure at day 5. Fibrin glues, particularly those containing a low concentration of fibrinogen, improved the bursting pressure in both ischemic (high–risk) and non–ischemic (low-risk) colon anastomosis. No significant differences were detected with respect to other markers of healing or anastomotic/abdominal complications.
The use of a uniform model with an objective method of quantifying anastomotic strength was one strength of our study. Bursting pressure is considered to be a valid indicator of anastomosis impermeability, particularly before day 7 (27). In this context, a lack of additional groups to describe the evolution of treated anastomosis over time may be considered as a limitation of our study, together with the fact that the validity of hydroxyproline measurements has been questioned (28).
Selective anastomotic devascularization impairs experimental anastomotic healing at day 7 in rats (29-30). This result is in agreement with a previous study that demonstrated a clear inverse correlation between leakage rate and tissue oxygen tension (31). In the present study, we chose to measure bursting pressure at day 5 based on previous experiments performed in our laboratory at post-anastomosis days 1-10 (n = 6 for each day). On days 1-4, all anastomoses burst at almost 0 mmHg. This reduced anastomotic strength at early time points is thought to be caused by collagen degradation by matrix metalloproteinases (32). At day 5, the bursting pressure was significant. In addition, in a clinical setting, anastomotic leakage is observed mostly between days 3 and 6 (33).
Some bursting occurred in the tissue adjacent to the anastomotic site rather than at the site itself. Therefore, it would seem logical to consider bursting pressure as an inadequate parameter for the evaluation of anastomotic strength. Nevertheless, it has been reported that the bursting pressures of both the anastomoses and the adjacent uninjured segments are almost the same from post-operative days 5-10 (34). The matrix in the bowel contains mainly collagen types I, III, and V, which are the major isoforms present during colon repair, with significant hydroxyproline concentrations at day 4 and maximal levels of this marker at day 7 (35). In addition, we found no correlation between the collagen content of the anastomoses and mechanical strength, as has been reported previously (36).
The lack of differences between groups with respect to microscopic parameters and collagen content is interesting. Some differences in the results between our work and other studies may be related to the nature of the high-risk model. There is a trend toward more inflammation and less angiogenesis and fibroblast proliferation in the ischemic colon, which is the model used in the current study. This lack of fibroblast infiltration may lead to reduced collagen content. A study reported that breaking strength was enhanced by metalloproteinase inhibitors at 3 days after colonic anastomosis and demonstrated that a loss of collagen content is associated with anastomotic weakening at this time point (32). Because our measurements were performed at day 5 and not later, as in other studies, we can speculate that the lack of differences may also be due to timing. This speculation is in accordance with findings in rat colon anastomoses with Tissucol©, in which collagen content was low at five days compared with seven days (37).
Fibrin glues may function to connect wound edges or may act as sealants covering and protecting the anastomosis (11). Based on our results, we believe that the higher bursting pressures observed with the diluted fibrin glue were a purely adhesive effect, with no modifications in the healing process at day 5 post-anastomosis. Although the thrombin concentration influences the coagulation time and tensile strength of fibrin glues (38), such effects were not observed within the parameter ranges studied here, and only a marginal effect of a lower thrombin concentration was observed in the high-risk group compared with the low-risk group. Senol et al. (39) found higher bursting pressures and hydroxyproline levels in high-risk colon anastomoses. In rats with peritonitis, compared with only sutures, using Tisseel VH®, a glue with a very high concentration of fibrinogen (75–115 mg/mL) and a low concentration of thrombin in the range that was used in our study (400–600 IU/mL).
No colon preparation was used in our model. Although infection has long been known to increase collagenolytic activity after colon anastomosis (36), it has been demonstrated that certain bacteria present in the normal intestinal flora have a positive influence on colon wound healing (40). Although no control for this parameter was performed, we consider the groups to be homogeneous and comparable.
Other researchers have determined that 5-fluorouracil has a negative impact on bursting pressure at day 8, and normalization of this parameter was noted after the use of fibrin (120 mg/mL) and thrombin (500 IU/mL) (Tissucol©) (41-43). This result may indicate the different advantages of sealants depending on the model used, as well as the inadequacy of defining only “high risk” with no consideration of the specific conditions responsible for the increased risk.
Making clinical decisions based on incomplete basic data is often difficult. Clearly, our demonstration that the concentrations of fibrin and thrombin in fibrin glues influence the anastomotic integrity in the colon does not imply that these glues should be used in high risk patients. However, we believe that these results suggest the need to continue evaluating these effects in the laboratory and in clinical settings.
In brief, a fibrin glue with a low fibrinogen concentration (40 mg/mL) and a high thrombin concentration (1000 IU/mL) provided stronger adherence in low-risk anastomoses, whereas a fibrin glue with a low fibrinogen concentration and either a high or low thrombin concentration normalized the bursting pressure of high-risk ischemic left-colon anastomoses in rats at post-operative day 5. All of this was not correlated with the collagen concentration or the macroscopic or histological appearance, which allowed us to conclude that the effects of a reduced fibrinogen concentration depend mainly on improved mechanical adherence. We believe that it is relevant to discriminate between the sealing, adhesive, or healing effects when evaluating any product to be applied in digestive-tube anastomoses.
ACKNOWLEDGMENTSThis work was supported in part by grants (SIMORELOS-1999-0302-009; FOFOI 2003/042) and by the author's own resources.
AUTHOR CONTRIBUTIONSPortilla-de Buen E, Orozco-Mosqueda A, Leal-Cortés C, Fuentes-Orozco C, Alvarez-Villaseñor AS, Macías-Amezcua MD, and González-Ojeda A participated in the protocol design and statistical analysis and were involved in editing as well as final critical revision of the manuscript. Portilla-de Buen E, Orozco-Mosqueda A, and Alvarez-Villaseñor AS participated in the surgical procedure and in the critical revision of the final version of the manuscript. Vázquez-Camacho G participated in the histological evaluation and in the critical revision of the final version of the manuscript.
No potential conflict of interest was reported.