To evaluate the effect of ginger extract on the expression of NFκB and TNF-α in liver cancer-induced rats.
METHODSMale Wistar rats were randomly divided into 5 groups based on diet: i) control (given normal rat chow), ii) olive oil, iii) ginger extract (100mg/kg body weight), iv) choline-deficient diet + 0.1% ethionine to induce liver cancer and v) choline-deficient diet + ginger extract (100mg/kg body weight). Tissue samples obtained at eight weeks were fixed with formalin and embedded in paraffin wax, followed by immunohistochemistry staining for NFκB and TNF-α.
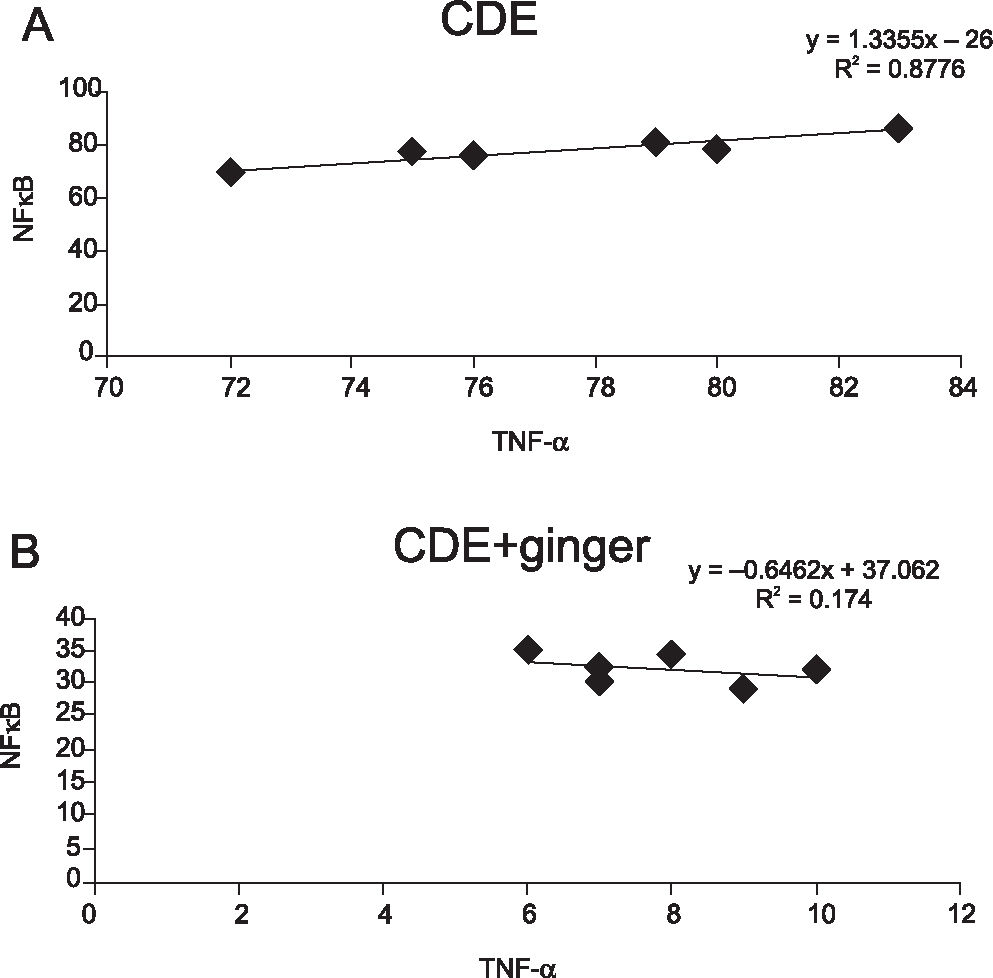
RESULTSThe expression of NFκB was detected in the choline-deficient diet group, with 88.3 ± 1.83% of samples showing positive staining, while in the choline-deficient diet supplemented with ginger group, the expression of NFκB was significantly reduced, to 32.35 ± 1.34% (p<0.05). In the choline-deficient diet group, 83.3 ± 4.52% of samples showed positive staining of TNF-α, which was significantly reduced to 7.94 ± 1.32% (p<0.05) when treated with ginger. There was a significant correlation demonstrated between NFκB and TNF-α in the choline-deficient diet group but not in the choline-deficient diet treated with ginger extract group.
CONCLUSIONIn conclusion, ginger extract significantly reduced the elevated expression of NFκB and TNF-α in rats with liver cancer. Ginger may act as an anti-cancer and anti-inflammatory agent by inactivating NFκB through the suppression of the pro-inflammatory TNF-α.
Inflammatory disorders such as gastritis, esophagitis, and hepatitis, which are caused not only by infectious agents such as viruses, bacteria and parasites but also by physical and chemical agents like heat, acid, cigarette smoke and foreign bodies, are recognized as risk factors for human cancer.1,2 Inflammation is considered to play an important role in the pathophysiology of cancer. However, when inflammation becomes chronic or lasts too long, it can be harmful. The diagnosis of inflammation and its biomarkers are not fully understood; however, pro-inflammatory cytokines, chemokines, adhesion molecules and the inflammatory enzymes have been linked to chronic inflammation.2
Chronic inflammation has been found to mediate a wide variety of diseases including cardiovascular diseases, diabetes, arthritis, Alzheimer’s disease, pulmonary diseases and autoimmune diseases. Chronic inflammation has also been associated with various steps involved in carcinogenesis as well as cellular transformation, promotion, survival, proliferation, invasion, angiogenesis and metastasis.2–4 During the carcinogenesis process, tumor-infiltrating inflammatory cells produce various types of cytokines. It has been proposed that pro-inflammatory cytokines including TNF-α, IL 1β, IL- 6 and interferon-γ contribute to carcinogenesis by influencing the survival, growth, mutation, proliferation, differentiation and movement of tumor cells.5 Many pro-inflammatory cytokines can activate the transcriptional factor NFκB, while some of the effects of pro-inflammatory cytokines may be mediated through the NFκB pathway.6–8
TNF-α is one of the pro-inflammatory cytokines and is a major inducer of NFκB. TNF-α has been implicated in tumor promotion in various experimental models of carcinogenesis.3,8 The incidence and multiplicity of papilloma in a two-stage mouse skin carcinogenesis model were found to be lowered in TNF-α −/− animals as compared with mice over-expressing TNF-α.9 TNF-α, interleukins, COX-2, and other chemokines can also be regulated by the transcriptional factor NFκB. Although this factor is expressed in an inactive state in most cells, cancer cells expressed an activated form of NFκB, which was induced by various inflammatory stimuli and carcinogens.10 Many studies have linked the NFκB signaling pathway and its regulation with the inflammatory response.10, 11 Thus, inhibiting the NFκB signaling pathway might be a therapeutic strategy in conjunction with the usage of chemopreventive agents such as ginger.12,13
Ginger has long been used in traditional medicine as a cure for some diseases including inflammatory diseases.14 Ginger contains active phenolic compounds such as gingerol, paradol and shogoal that have antioxidant,15 anti-cancer,16 anti-inflammatory,17 anti-angiogenesis18 and anti-artherosclerotic properties.19 It has also been shown to down-regulate NF-κB-regulated gene products involved in cellular proliferation and angiogenesis, including IL-8,20 VEGF21 and ovarian cancer cells.22
In the present study, we tested the potential anti-inflammatory and anti-cancer effects of ginger extract by using an immunohistochemistry technique to detect the presence of the inflammatory marker TNF-α and the transcription factor NFκB and to observe the correlation between these two factors in liver carcinogenesis.
MATERIALS AND METHODSAnimals, Chemicals and TreatmentMale Wistar albino rats aged 3–4 months and weighing 200–250 g were supplied by the Animal Care Unit of Universiti Kebangsaan Malaysia (UKM), Kuala Lumpur, Malaysia. The study was approved by the Animal Ethics of Faculty of Medicine, Faculty of Medicine, UKM. Animals were kept in pairs in polycarbonate cages and provided with food and water ad libitum. They were maintained under standard conditions of temperature and humidity with an alternating light and dark cycle. Rats were randomized into five groups of six animals each. The first group and the second group served as control groups and were fed with normal rat chow (Gold Coin, Malaysia) and olive oil, respectively. The latter served as a control for the gavage method and the delivery of ginger. Rats in group 3 received ginger extract at 100 mg/kg body weight by the gavage method. Ginger extract was prepared by ethanol extraction and kept at 4°C. It was dissolved in olive oil and force-fed to the rats. Rats in group 4 were fed with a choline-deficient diet (ICN Biochemicals, USA) plus 0.1 % ethionine (Sigma Chemical Co., USA) in drinking water; this is known as the CDE diet. This is the model to induce the production of oval cells, which are the precursor cells of liver cancer23. Rats in group 5 received ginger as in group 3 plus the CDE diet. All rats were killed at eight weeks for the observation of liver tumor incidence, and the liver tissues were excised after perfusion and embedded in paraffin blocks for immunohistochemistry staining.
Liver perfusion and preparation for paraffin blocksThe rats were sacrificed using ether. All equipments for dissection were sterilized using 70% alcohol before use. The rats were anesthetized intraperitoneally with Zoletil 50 (0.1 ml/100g body weight), followed by heparin (25, 000 U/ml) injection to the inferior vena cava. The portal vein was then canulated using an intravenous catheter, size 16 G (2.25 inches) for the perfusion procedure. The liver was then perfused in PBS, pH 7.4, for one minute at a 10ml/min flow rate at room temperature, followed by 1:1 ratio of 4% paraformaldehyde and 0.1% glutaraldehyde for three minutes. Then the liver was perfused in PBS for another two minutes. A portion of the perfused liver was then immersed in 10% formalin for fixation before embedding in paraffin.
Preparation of tissues sectionsThe paraffin-embedded tissues were cut 3μm thick with Leica RM2135 rotation microtome (Leica, Germany). The tissue sections were placed on poly-L-Lysine (Sigma-Aldrich Co. USA)-treated slides with 1:10 dilution. The slides were then dried overnight and stored at room temperature until being used for staining.
General protocol for immunohistochemistry stainingThe sections were deparaffinized and hydrated by sequential immersion in xylene, graded alcohol solutions (100%, 95%, 80% and 70%) for three minutes at each concentration and running water for three minutes. Then sections were incubated in 3% hydrogen peroxide for 10 min to block the activity of endogenous peroxidases. Sections were washed with TRIS-HCl-buffered saline (TBS) before immersion in Target Retrieval Solution (TRS) (DAKO, U.S.A.) for 20 minutes in a water bath at 98°C. The TRS solution could be either in high pH, where it ranged from 9.8 to 9.9 or low pH, with a range of 6.0 to 6.2. The slides with TRS solution were left at room temperature for 20 minutes before being washed with TBS three times for three minutes each. To reduce the background staining, the slides were immersed in Biotin and avidin solution for 30 minutes. The sections were then further blocked with Bovine Serum Albumin (BSA) to reduce the non-specific staining, followed by one hour of incubation with the primary antibody at different concentrations (1:50, 1:100, 1:200, 1: 400, 1:500, 1:600 and 1:1000) at room temperature or overnight at 4°C. The sections were washed three times with TBS for three minutes each before incubating for 30 minutes with the secondary antibody conjugated with biotin and subsequently with Streptavidin-HRP (anti-mouse, anti-rabbit and anti-rat LSAB + System-HRP kit, DAKO, USA). The sections were washed again with TBS before finally incubating with DAB for 10 minutes. The sections were counterstained with Meyer’s Hematoxylin and mounted with DPX for viewing of the slides.
NFκB immunohistochemistry stainingCervical cancer tissues obtained from the Department of Pathology, University Kebangsaan Malaysia Medical Center (UKMMC), served as the positive and negative controls. The optimum concentration of the primary antibody (polyclonal rabbit anti-human Nuclear Factor kappa B, Santa Cruz Biotechnology, Inc.) for NFκB was found to be 1:500 for one hour of incubation at room temperature using low pH Target Retrieval Solution (TRS) (DAKO, USA).
TNF-α immunohistochemistry stainingBreast cancer tissues obtained from the Department of Pathology, University Kebangsaan Malaysia Medical Center (UKMMC), served as the positive and negative controls. The optimum concentration of the primary antibody (polyclonal rabbit anti-mouse Tumour Necrosis Factor-alpha, Hycult Biotechnology) for TNF-α was found to be 1:100 for one hour of incubation at room temperature using high pH Target Retrieval Solution (TRS) (DAKO, USA).
Immunostaining analysisImmunoreactivity evaluation was based on percentage of positive staining of NFκB and TNF-α. The mean percentage of positive-staining cells was determined by counting 1000 stained cells at 10 different fields observed under 400X magnification using a light microscope.
Statistical analysisData obtained were analyzed using the SPSS 11.0 program. Results are presented as mean ± SD. The one-way ANOVA test was used, with p <0.05 regarded as significant. Pearson correlation analysis was carried out to determine the relationship between NFκB and TNF-α in both the CDE and the CDE-treated ginger group.
RESULTSLiver tumor incidence and body weights of ratsTable 1 summarizes the incidence of liver neoplasms in rats on a low lipotrope diet with ethionine in drinking water and the effect of ginger on cancer incidence. There were no liver nodules in either the control groups (normal rat chow and olive oil) or the ginger treated rats. However, in CDE rats the preneoplastic nodules incidence was 100% and the average size of the nodules was 0.5–1 cm. Ginger treatment to CDE rats lowered the liver nodules incidence to 17%.
Effect of Ginger on the incidence of liver neoplasms in ethionine induced rat hepatocarcinogenesis. The formation of preneoplastic liver nodules was induced in rats by feeding them with a choline-deficient diet supplemented with 0.1 ethionine in drinking water. Ginger extract at 100 mg/kg body weight (dissolved in olive oil) was force-fed to rats. Rats were killed at eight weeks, as described in the Materials and Method section, for the observation of liver nodules
Table 2 summarizes the body weights of rats under different diet conditions. With the exception of the CDE group, all other groups showed significant elevations of body weight at eight weeks compared with those at the 0 week. However, CDE rats showed reduced body weights due to the carcinogen diet, which had altered the rats’ metabolism and reduced their appetite for food.
Weight of rats with different diet treatment
| Diet treatment | Week 0 body wt (g) | Week 8 body wt (g)* |
|---|---|---|
| Control | 238.11 ± 5.44 | 313.73 ± 7.77 |
| CDE | 240.21 ± 2.19 | 222.41 ± 4.76 |
| Olive oil | 239.59 ± 4.52 | 300.58 ± 10.89a |
| Ginger (100mg/kg body wt) | 239.58 ± 4.52 | 303.10 ± 6.27a |
| CDE + Ginger (100mg/kg body wt) | 239.74 ± 2.23 | 259.96 ± 9.09a |
Data shows Mean ± S.D.;
Figure 1A shows the normal morphology of hepatocytes, Kupfer cells and sinusoids lining the control group, while Figure 1B showed abnormal morphology of liver tissues in the CDE group with the presence of numerous oval cells (scanty cytoplasm with large nucleus), which are precursors of liver cancer cells.
H & E staining on liver tissue of (A) control group (given a normal diet) (B) CDE group without treatment of CV (liver cancer was induced with 0.1% ethionine in drinking water plus a choline-deficient diet). H: Hepatocyte, K: Kuppfer cell, PV: Portal vein, S: Sinusoid, O: Oval cell (magnification ×400)
Figure 2a showed positive NFkB staining in the control cervical cancer tissue. Figures 2b and 2c show the control group and the group of rats supplemented with ginger extract. Neither group showed any expression of NFκB. However, the liver cancer-induced group (CDE)(Figure 2d, Figure 3) showed increased expression of NFκB (88.3%), while the ginger extract-supplemented group showed significantly reduced expression (32.4%, p < 0.05) (Figure 2e, Figure 3).
Immunohistochemical expression of NFkB. (A) Positive NFκB staining in cervical cancer tissue at optimum concentration of 1:500, (B) Control group (C) Ginger extract group, (D) Choline deficient diet with ethionine in drinking water, CDE group and (E) CDE + ginger extract (100 mg/kg body wt) group. The arrows indicate positive staining of NFkB (400x). OV: oval cells; H: hepatocytes; S: sinosoids; VP; vena portal (x400)
The effect of ginger extract on NFkB expression in liver cancer-induced rats. The graph shows the positive expression of NFkB based on the different diets given. A = p ≤ 0.05 compared with eight-week control group; b = p ≤ 0.05 compared with olive oil group; c = p ≤ 0.05 compared with ginger group; d = p ≤ 0.05 compared with liver cancer induced group (CDE); e = p ≤ 0.05 compared with CDE+ginger group
Figure 4a showed positive staining for TNF-α. in the positive control of breast cancer tissue. Figures 4b and 4c show the control group and the group of rats supplemented with ginger extract. Neither group showed any expression of TNF-α. However, the liver cancer-induced group (CDE) (Figure 4d, Figure 5) showed increased expression of TNF-α (83.3%), which was reduced significantly (7.9%, p < 0.05) with ginger extract supplementation (Figure 4e, Figure 5).
Immunohistochemical expression of TNF-α. (A) Positive control for TNF-α staining in breast cancer tissue at optimum concentration of 1:100; (B) Control group; (C) Ginger extract group; (D) CDE group; and (E) CDE + ginger extract group. The arrows indicate positive staining of TNF-α (400x). OV: oval cells; H: hepatocytes; S: sinosoids; VP; vena portal
The effect of ginger extract on TNF-α expression in rats with induced liver cancer. The graph shows the positive expression of TNF-α based on the different diets given. a = p ≤ 0.05 compared with eight-week control group; b = p ≤ 0.05 compared with olive oil group; c = p ≤ 0.05 compared with ginger group; d = p ≤ 0.05 compared with CDE group; e = p ≤ 0.05 compared with CDE+ginger group
There was a significant correlation (r = 0.8776) demonstrated between NFκB and TNF-α in the CDE group (p < 0.01) but not in the CDE + ginger group (Figures 6a and 6b, respectively).
Correlation coefficient between NFkB and TNF-α expressions in (A) CDE group and in (B) CDE supplemented with ginger extract (100 mg/kg body weight) group. Significant correlation was observed between NFkB and TNF-α in CDE group (r = 0.8776, p < 0.01) but not in the CDE group treated with ginger extract
There are numerous lines of evidence that suggest that nuclear factor NFκB, a pro-inflammatory transcription factor, could promote tumorigenesis after being activated by inflammatory agents, carcinogens and tumor promoters.7 It is constitutively active in most tumor cells, and its suppression in these cells leads to inhibition of proliferation, arrest of cell cycle and apoptosis.24 In the last few years, work from several groups of researchers provided a causal link between active constitution of NFκB and liver neoplastic progression.25 Hepatocellular carcinoma (HCC) is the fifth most common malignancy in the world and is the third most common cause of cancer-related death worldwide.26
Accumulating evidence suggests that many dietary factors may be used alone or in combination with traditional chemotherapeutic agents to prevent or treat cancer. The main advantage of using natural or dietary compounds as an anti-cancer remedy is that they seem to have low toxicity and show very few adverse side effects. Recently, some plant products have been studied for their possible action as inhibitors of the NFkB pathway. Ginger (Zingiber officinale) is widely used all over the world as a spice and condiment in daily cooking. It is a natural food component with many active phenolic compounds such as shagaol and gingerol, and it has been shown to have anti-cancer and antioxidant effects.12 We have shown here that ginger extract was able to reduce the incidence of liver neoplasms in rats; in addition, to our knowledge, this is the first study reporting that the anti-cancer effect exhibited by ginger on liver cancer cells is mediated by inflammatory markers NFκB and TNF-α. Oval cell proliferation precedes neoplasia in many rodent models of hepatocellular carcinoma, and prevention of this proliferative response can reduce the risk of subsequent carcinoma.23
We have shown here that ginger extract was able to block the elevated expression of NFκB in liver cancer-induced rats. Similarly, elevated expression of TNF-α in liver cancer rats was also blocked when treated with ginger extract (100mg/kg body weight). It is apparent that ginger may act as an anti-cancer and anti-inflammatory agent by blocking the activation of NFκB via the suppression of pro-inflammatory cytokine, TNF-α.17 Other, similar reports have also shown the inhibitory effect of ginger on the NFκB pathway: topical application of 6-gingerol inhibited TPA-induced COX-2 expression and suppressed NFκB DNA binding activity in mice skin.13, 27 The natural active compounds in ginger (gingerols and zerumbone) have been found to be potent inhibitors for NFκB and pro-inflammatory cytokine TNF-α. Ginger may block any one or more steps in the NFκB signaling pathway, such as the signals that activate the NFκB signaling cascade, translocation of NFκB into the nucleus, DNA binding of dimers or interactions with the basal transcriptional machinery.28 Inhibiting the activity of NFκB, will subsequently inhibit growth of tumor cells and block metastasis and angiogenesis. The 6-gingerol and 6-paradol have been reported to possess a strong anti-inflammatory activity and to suppress the TNF-α production in TPA-treated female ICR-mice and rats.12, 27
The activation of the TNF-α gene causes the release of pro-inflammatory cytokines, and this would activate the transcriptional factor NFκB. Activation of NFκB would activate the expression of other inflammatory cytokines such as COX-2, LOX-2, other chemokines and iNOS, which would lead to carcinogenesis.29 Although no significant correlation between NFκB and TNF-α was found in CDE rats treated with ginger extract, we did show a significant correlation of these two inflammatory markers in rats induced with liver cancer. A possible explanation could be the few samples (12 slides) representing each treatment.
In conclusion, we have shown here that in liver cancer cells, NF-κB is constitutively activated and that blocking NFκB activation with ginger resulted in suppressed production of NFκB and TNF-α. This is in line with findings that many of the pathways that mediate adaptive survival strategies in cancer cells are under the transcriptional control of NFκB.30 Thus, the ginger extract may have a chemotherapeutic effect in the treatment of liver cancer.
We wish to thank the Department of Biochemistry, Faculty of Medicine, University Kebangsaan Malaysia Medical Centre (UKMMC), for financial support.