To analyze and compare the evolution of hematological parameters and body iron content between exclusively breastfed late-preterm and term newborns during the first two months of life.
METHODS:Cohort study. Weight, length, head circumference, body mass index, hemoglobin, hematocrit, reticulocytes, total iron-binding capacity, transferrin saturation, serum iron and ferritin were measured in 25 late-preterm and 21 term newborns (at birth and at one and two months of age) who were exclusively breastfed. Statistical analysis: Kolmogorov-Smirnov test, one-way ANOVA or Kruskal-Wallis test; and Student's t-test or Mann-Whitney test. Significance: p<0.05.
RESULTS:The corrected gestational ages of the late-preterm infants were 39.98 weeks at one month of life and 44.53 weeks at two months. Anthropometric measures and the body mass index increased over time (p<0.001) and hemoglobin, hematocrit, reticulocytes and body iron content decreased (p<0.001). Late-preterm infants at term corrected gestational age had reduced hemoglobin, hematocrit and reticulocyte concentrations, and reduced total iron-binding capacity (p<0.001) and serum iron (p = 0.0034) compared with values observed in term newborns at birth. Late-preterm newborns at a corrected gestational age of one month post-term had hemoglobin (p = 0.0002), hematocrit (p = 0.0008), iron (p<0.0001) and transferrin saturation (p<0.001) levels lower than those of term newborns at one month of age and a higher total iron-binding capacity (p = 0.0018). Ferritin did not differ between the groups.
CONCLUSION:Exclusively breastfed late-preterm newborns presented greater reductions in hemoglobin/hematocrit and lower iron stores at a corrected gestational age of one month post-term than did term newborns, suggesting specific iron supplementation needs.
Iron is a trace element found at high levels in the human body (1) that participates in various metabolic pathways, especially erythropoiesis and neurodevelopment (2). Modifications in these reactions can modify sensorimotor, cognitive/language, social/emotional and psychological/behavioral activities during brain development in newborns (2).
Approximately 80% of fetal iron storage is deposited during the third trimester of gestation (3); this storage is fundamental to the formation of hemoglobin (Hb), which is responsible for transporting oxygen to tissues. Hb levels in the fetus increase during pregnancy, with high concentrations observed at birth. However, after birth, polycythemic conditions, a decrease in fetal Hb, an increase in adult-type Hb, higher environmental oxygen concentrations and Hb oxygen saturation promote great tissue oxygenation and thus decrease the stimulation of erythropoietin production (4), reducing erythrocyte release.
Therefore, the Hb level is reduced by approximately 30 to 50%, reaching a nadir at six to twelve weeks after birth in term infants and one to four weeks earlier in preterm infants (5). Certain conditions may explain this difference, such as frequent blood sampling for laboratory tests; an immature erythropoietic response; low sensitivity of hypoxemia sensors; folate, vitamin B12 and E (antioxidant erythrocyte) deficiencies; and a volume expansion of the blood cell mass due to rapid postnatal growth (6).
Considering that preterm infants are born before their fetal storage is complete, they may develop an increased risk of iron deficiency and even anemia during the early postnatal period (7).
Late-preterm (LPT) newborns, despite appearing healthy, are premature and carry all of the inherent risks, such as increased difficulty in the postnatal transition and a greater risk of neonatal morbidity compared with term newborns, in addition to a higher incidence of readmissions (approximately two times higher) and neonatal mortality (8). This risk is reflected in the neonatal mortality rates of LPT newborns and term newborns, which were 7.7/1000 live births and 2.5/1000 live births, respectively, in 2002 in the United States (8). Additionally, the long-term effects of late prematurity suggest higher morbidity associated with neurodevelopment, with delayed brain development in early childhood, cerebral palsy and low academic proficiency (9).
Evidence available in the literature indicates that exclusively breastfed term newborns have sufficient hepatic iron reserves for growth and development and do not require supplementation before the sixth month of life. LPT newborns may be born with lower nutrient reserves, including total body iron, which can influence postnatal hematological evolution, requiring iron treatment or replacement. Based on this information, a study was developed to analyze and compare the evolution of hematological parameters and body iron content between exclusively breastfed LPT and term newborns during the first two months of life.
METHODSA cohort study was carried out between March 2009 and December 2011 at a tertiary public hospital in São Paulo, Brazil and approved by the Ethics Committee for Research Analysis (CAPPesq) of the Clinical Board of the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo.
In total, 21 term newborns (gestational age (GA) between 37 weeks and 41 weeks, 6 days) (10) and 25 LPT newborns (GA between 34 weeks and 36 weeks, 6 days) (11) who were exclusively breastfed were included. Informed consent forms for the study were signed by legally authorized representatives.
The exclusion criteria comprised congenital malformations; genetic abnormalities and congenital or perinatal infections; twins; Apgar scores ≤3 at one minute or ≤7 at five minutes; newborns under the 10th and above the 90th percentile according to the curve reported by Alexander et al. (12); fasting; blood transfusion; hemolytic anemia; maternal diseases or complications that could interfere with iron metabolism, such as malnutrition, heart disease, skin disorders, lung diseases, gastrointestinal diseases, acute infection during childbirth or within ten days before birth, hemoglobinopathies, diabetes and hypo/hyperthyroidism; and maternal smoking or drug use.
The infants were divided into two groups: Group 1 (G1) included LPT newborns and Group 2 (G2) included term newborns.
Blood samples were collected at birth (from the umbilical cord) and at one month and two months of age. The at-birth blood sample was collected after umbilical cord clamping within the first 30 seconds after birth. Anthropometric measurements (weight, length and head circumference) were performed and the body mass index (BMI) was calculated within the first 24 hours of life and at the chronological ages of one month and two months of age for term newborns and at corrected GAs of term and one month post-term (post-T) for LPT newborns. All anthropometric measurements were performed by the researchers, including blood sample collections. All infants were followed at the specialized outpatient department of the same hospital. The parents were asked about social factors, breastfeeding techniques and medication taken by the child or by the mother. The mothers received 300 mg of iron supplementation for two months after delivery. The infants started receiving iron supplementation (2 mg per kilogram per day) after two months of age. When Hb levels or iron stores were found to be low at one month of life, those patients were excluded and their iron-deficiency anemia was treated.
Laboratory tests were conducted at the Laboratório de Análises Clínicas do Instituto da Criança (Clinical Analysis Laboratory of the Child Institute of the Faculdade de Medicina da Universidade de São Paulo, Brazil). Hb, hematocrit (Ht) and reticulocyte tests were performed using the Roche Diagnostics Sysmex XT-2000i Automated Hematology Analyzer. The ferritin levels (immunoturbidimetric method), serum iron (Guanidine/FerroZine® method) and total iron-binding capacity (TIBC) (automated method) were evaluated using the Siemens Dimension RxL system. Transferrin saturation (TfSat) was calculated using the following equation: iron/TIBC.
Statistical analysisThe sample size comprised 20 infants in each group and was chosen based on the assumption that the groups to be compared would have an approximate difference of 30%, using α = 5% and a power of 80%. The mean values and standard deviations found in the literature for Hb (15.2±1.5 g/dl) (13) and ferritin (173±59 ng/ml) (14) in term and LPT newborns were considered and differences of 1.5 g/dl for Hb and 59 ng/ml for ferritin between the groups were deemed necessary for significance. The definition of anemia for statistical analysis was based on Hb levels below 10.5 g/dl (15,16).
The results are reported as means and standard deviations. The Kolmogorov-Smirnov test was used to evaluate normality. The Kruskal-Wallis test was used to evaluate differences when the distribution was not normal. A homogeneity test was used to check the equality of variances between the groups when the distribution was considered normal. One-way analysis of variance (ANOVA) was performed when the distribution was considered normal. Otherwise, the robust Brown-Forsythe test for equality of means was applied to evaluate differences (p<0.05). The comparison of means was performed using Student's t-test or the Mann-Whitney test. A box plot was used to analyze the distribution of the Hb values at term and one month post-T and the differences between groups. The results were considered statistically significant if p<0.05. SPSS v17 software (Statistical Package for the Social Sciences version 17.0, SPSS Inc., Chicago, IL, 1999) and GraphPad InStat (version 3.10, GraphPad Software Inc., La Jolla, CA) were used for data processing.
RESULTSThere were no differences between the groups regarding maternal characteristics (Table1), except that pregnancy complications were more frequent in the LPT group (p<0.001), especially premature labor and amniorrhexis.
Population characteristics of late-preterm and term newborns.
| G1 (LPT) | G2 (term) | p | |
|---|---|---|---|
| n = 25 | n = 21 | ||
| Mother's age | 25.96±8.37 | 24.00±7.40 | 0.337 |
| First pregnancy | 12 (48%) | 11 (52.38%) | 0.471 |
| Cesarean section | 9 (36%) | 9 (42.86%) | 0.417 |
| Disease/complications | 36 | 6 | <0.001 |
| Preterm labor | 19 | 0 | <0.001 |
| Premature amniorrhexis | 4 | 1 | 0.116 |
| Gestational age (weeks) | 35.67±0.92 | 39.75±1.01 | <0.001 |
| Male (%) | 16 (64%) | 11 (52.38%) | 0.370 |
| Apgar 1st minute>4 and 5th minute>6 | 25 (100%) | 21 (100%) | - |
| Age at first return (days) | 30.46±1.74 (n = 24) | 30.52±2.18 (n = 21) | 0.830 |
| Age at second return (days) | 61.62±3.12 (n = 21) | 61.33±1.32 (n = 21) | 0.565 |
| Corrected GA at first return (weeks) | 39.98±0.95 | - | - |
| Corrected GA at second return (weeks) | 44.53±1.11 | - | - |
GA: Gestational age; LPT: Late preterm.
Regarding the characteristics of the newborns (Table1), all of them had early umbilical cord clamping (<30 seconds). The mean GA at birth was 35.67±0.92 weeks among the LPT newborns and 39.7±1.01 weeks among the term newborns, with a significant difference between the groups (p<0.001). LPT newborns had corrected GAs of 39.98 weeks at one month of life and 44.53 weeks at two months.
All infants showed increases in weight, length, head circumference and BMI (p<0.001) (Table2), although these measures were lower in preterm infants. However, LPT infants at term corrected GA had a greater length and head circumference (p = 0.0043 and p<0.001, respectively) than did term newborns at birth and at one month post-T, as well as a lower BMI (p = 0.0013) than that of term newborns at birth. Only the head circumference of the LPT infants was greater than that of the term infants at one month of age (p = 0.0028).
Weight, length, head circumference and body mass index of late-preterm and term newborns during the first two months of life, with the corrected gestational age for the late-preterm newborns.
| Weight (g) | G1 (LPT) | G2 (term) | p | p |
| At birth | 2603.00±357.56 | 3404.00±347.75 | <0.001 | - |
| 1 month/term* | 3225.83±514.09 | 4305.71±458.25 | <0.001 | 0.2502† |
| 2 months/1 month post-T** | 4461.43±575.74 | 5426.43±638.10 | <0.001 | 0.3249†† |
| p♦ | <0.001 | <0.001 | ||
| Length (cm) | G1 (LPT) | G2 (term) | p | p |
| At birth | 45.48±1.97 | 48.71±1.02 | <0.001 | - |
| 1 month/term* | 50.10±1.85 | 53.90±1.59 | <0.001 | 0.0043† |
| 2 months/1 month post-T** | 54.14±1.70 | 57.86±1.46 | <0.001 | 0.6444†† |
| p♦ | <0.001 | <0.001 | ||
| HC (cm) | G1 (LPT) | G2 (term) | p | p |
| At birth | 32.80±1.06 | 34.57±0.90 | <0.001 | - |
| 1 month/term* | 35.60±1.02 | 37.57±0.97 | <0.001 | 0.0010† |
| 2 months/1 month post-T** | 38.38±0.84 | 39.43±1.09 | <0.001 | 0.0028†† |
| p♦ | <0.001 | <0.001 | ||
| BMI (kg/m2) | G1 (LPT) | G2 (term) | p | p |
| At birth | 12.54±1.10 | 14.34±1.30 | <0.001 | - |
| 1 month/term* | 12.80±1.50 | 14.79±1.01 | <0.001 | 0.0013† |
| 2 months/1 month post-T** | 15.20±1.65 | 16.19±1.60 | <0.001 | 0.3199†† |
| p♦ | <0.001 | <0.001 |
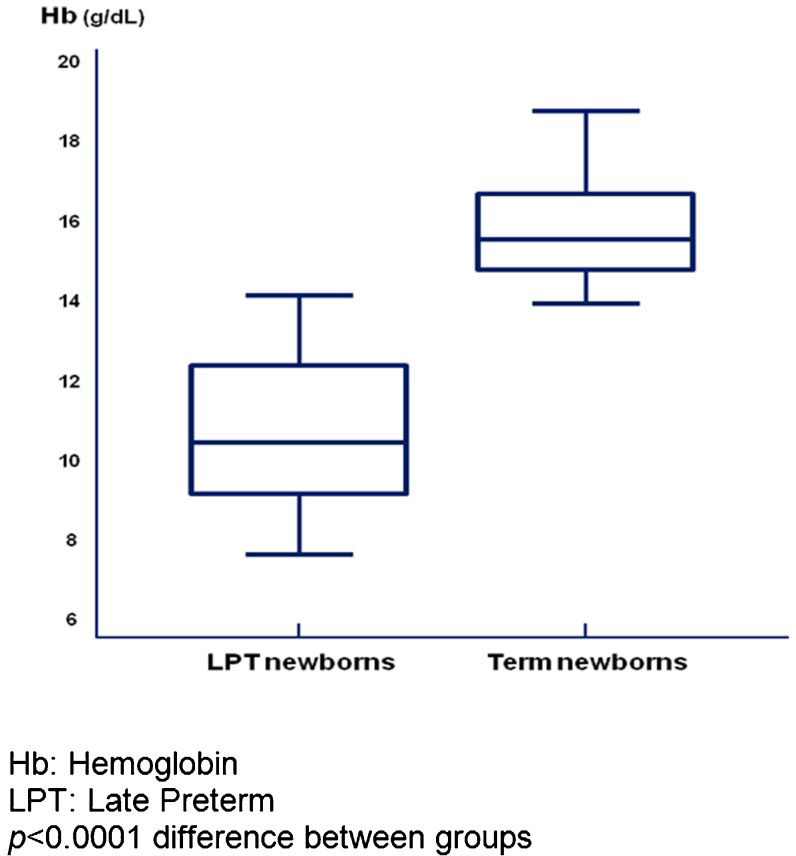
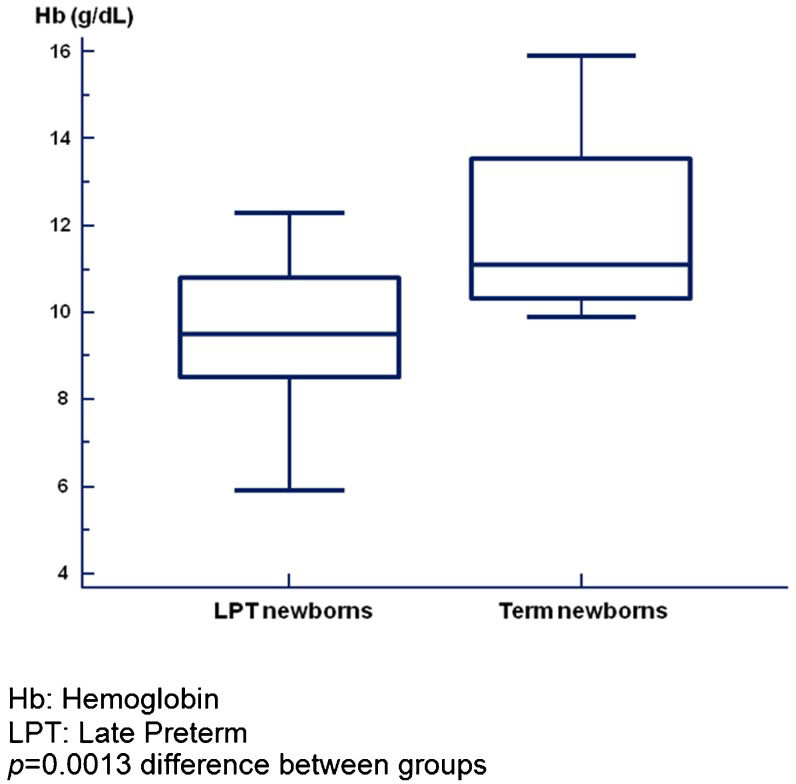
Hb and Ht (Table3) mainly decreased in the LPT newborns (p<0.001). At birth, a difference between the two groups was observed only for Ht (p = 0.010), whereas at two months, more dramatic differences in both Hb (p = 0.020) and Ht (p<0.001) were present. At corrected GAs of both term and one month post-T, the concentrations of Hb and Ht in the LPT newborns were lower than those in the term newborns (Table3). Box plot analysis indicated greater dispersion values for LPT newborns at term corrected GA than for term newborns at birth, whereas the dispersion was greater for term newborns at one month of age than for LPT newborns at a corrected GA of one month (Figures1 and 2). In both analyses, the median Hb values were higher in term newborns compared with LPT newborns at either term corrected GA (p<0.001) or a corrected GA of one month post-T (p = 0.0013).
Hemoglobin, hematocrit and reticulocytes in late-preterm and term newborns during the first two months of life, with the corrected gestational age for the late-preterm newborns.
| Hb (g/dl) | G1 (LPT) | G2 (term) | p | p |
| At birth | 15.70±1.80 | 15.96±1.40 | 0.600 | - |
| 1 month/term* | 10.83±1.84 | 11.95±1.96 | 0.060 | <0.001† |
| 2 months/1 month post-T** | 9.59±1.50 | 10.55±0.90 | 0.020 | 0.002†† |
| p♦ | <0.001 | <0.001 | ||
| Ht (%) | G1 (LPT) | G2 (term) | p | p |
| At birth | 44.21±5.22 | 48.60±5.04 | 0.010 | - |
| 1 month/term* | 30.45±5.00 | 33.58±5.46 | 0.060 | <0.001† |
| 2 months/1 month post-T** | 27.04±3.86 | 30.55±2.52 | <0.001 | 0.008†† |
| p♦ | <0.001 | <0.001 | ||
| Ret (%) | G1 (LPT) | G2 (term) | p | p |
| At birth | 5.34±1.18 | 3.34±1.25 | <0.001 | - |
| 1 month/term* | 1.71±1.18 | 0.79±0.40 | <0.001 | <0.001† |
| 2 months/1 month post-T** | 2.40±0.90 | 1.40±0.85 | <0.001 | <0.001†† |
| p♦ | <0.001 | <0.001 |
The incidence of anemia was 66.67% in LPT newborns at a corrected GA of one month post-T and 41.18% in term newborns at one month of age (p = 0.02).
Reticulocyte concentrations (Table3) presented similar variations in the two groups, decreasing at one month and increasing at two months. These concentrations were also higher in the LPT newborns throughout the study period. The reticulocyte concentrations in the term newborns were higher than those in the LPT newborns at term corrected GA and lower than those in the LPT newborns at a corrected GA of one month post-T (p<0.001).
All markers of iron stores varied throughout the two months (Table4). In LPT newborns, serum iron increased at one month and declined at two months (p<0.001). There were differences between the groups at birth (p<0.001) and at two months of age (p = 0.020) and lower concentrations were observed in the LPT newborns. The iron concentrations in the LPT newborns were lower at term corrected GA (p = 0.0034) and at a corrected GA of one month post-T (p<0.001).
Serum iron, total iron-binding capacity, ferritin and transferrin saturation in late-preterm and term newborns during the first two months of life, with a corrected gestational age for the late-preterm newborns.
| Serum iron (µg/dl) | G1 (LPT) | G2 (term) | p | p |
| At birth | 49.17±26.12 | 133.95±58.90 | <0.001 | - |
| 1 month/term* | 86.83±20.16 | 93.47±24.12 | 0.330 | 0.0034† |
| 2 months/1 month post-T** | 58.24±22.48 | 75.89±25.31 | 0.020 | <0.001†† |
| p♦ | <0.001 | <0.001 | ||
| TIBC (µg/dl) | G1 (LPT) | G2 (term) | p | p |
| At birth | 209.08±47.66 | 280.19±63.38 | <0.001 | - |
| 1 month/term* | 211.58±59.48 | 241.89±42.78 | 0.070 | 0.0010† |
| 2 months/1 month post-T** | 297.52±63.70 | 301.00±61.48 | 0.860 | 0.0018†† |
| p♦ | <0.001 | <0.001 | ||
| TfSat (%) | G1 (LPT) | G2 (term) | p | p |
| At birth | 24.79±16.06 | 47.69±17.54 | <0.001 | - |
| 1 month/term* | 44.12±17.27 | 39.77±11.93 | 0.360 | 0.5169† |
| 2 months/1 month post-T** | 20.94±10.52 | 26.94±12.09 | 0.100 | <0.001†† |
| p♦ | <0.001 | <0.001 | ||
| Ferritin (µg/l) | G1 (LPT) | G2 (Term) | p | p |
| At birth | 215.96±132.56 | 167.86±112.71 | 0.200 | - |
| 1 month/term* | 226.96±119.95 | 232.11±136.64 | 0.900 | 0.1150† |
| 2 months/1 month post-T** | 152.10±109.66 | 157.05±95.83 | 0.880 | 0.0529†† |
| p♦ | <0.001 | <0.001 |
TIBC increased over time (p<0.001). Lower levels were measured in the LPT newborns at birth (p<0.001) and at term corrected GA (p = 0.0001), although the levels were higher at a corrected GA of one month post-T (p = 0.0018).
TfSat decreased in a pattern similar to that of the TIBC. TfSat was lower in LPT newborns at birth (p<0.001) and at a corrected GA of one month post-T (p<0.001).
Ferritin changed over time and no differences were observed between the groups at any time point in the study, even when corrected GA was considered. Nevertheless, it was observed that the difference approached statistical significance (p = 0.0529) at one month post-T in LPT newborns compared with term newborns at one month of age.
DISCUSSIONAnalysis of the hematological parameters and iron content in exclusively breastfed term and LPT newborns during the first two months of life revealed greater reductions in Hb and Ht concentrations and smaller reserves of iron in the LPT newborns until a corrected GA of one month compared with the term newborns. These results suggest that LPT newborns have increased iron needs after birth.
The fact that the study groups were homogeneous with regard to maternal characteristics, particularly age, parity, prenatal care and the incidence of cesarean section, support the relevance of the above-mentioned differences. The higher frequency of pregnancy complications in the LPT newborn group, including premature labor and amniorrhexis, could be considered as expected events that contributed toward the early interruption of pregnancy.
In the same way, both groups only differed with respect to GA, which was significantly lower in the LPT newborns compared with the term newborns (p<0.001), as expected from the study design. The corrected GA of the LPT newborns at one month of life was 39.77±1.30 weeks, which was close to the GA of the term newborns at birth, whereas at two months of age, the LPT newborns had a corrected GA of one month post-T.
During postnatal development, all infants exhibited increases in weight, length, head circumference and BMI (p<0.001) at an expected rate and it can therefore be concluded that they received enough breast milk to meet their needs. An analysis based on the corrected GA revealed significant growth in the length and head circumference of both groups, although the LPT measurements were higher at term corrected GA than in the term newborns at birth. Only head circumference remained higher at a corrected GA of one month post-T than in the term newborns (p = 0.0028). This fact indicated that the LPT newborns must have achieved excellent growth to have reached a weight at term corrected GA similar to that of the term newborns at birth and at one month of life. Head circumference was greater than in term newborns during both assessments, indicating favorable growth of the central nervous system.
The more intense reduction in Hb and Ht and the increase in reticulocytes observed during all assessments (p<0.001) in the LPT newborns might signal the presence of intense bone marrow stimulation for erythropoiesis, as is expected at this corrected GA.
Analysis of the reductions in Hb indicated a high incidence of anemia in the LPT newborns. This finding reinforced the intense reduction in Hb observed in these newborns and the consequent risk of anemia.
The median Hb value observed in term newborns exceeded the 75th percentile of LPT newborns at each corrected GA in the study. Although these differences decreased from term corrected GA to a corrected GA of one month post-T, this difference could have been a consequence of the earlier nadir of anemia in LPT newborns, as is commonly observed during the first months of life and their recovery.
Early umbilical cord clamping, less than 30 seconds after birth, was likely an additional factor contributing to the high incidence of anemia observed in both groups (17).
Regarding body iron content markers, they were modified in both groups over time (p<0.001). Considering the corrected GA, LPT newborns at term and one month post-T had lower levels than those of term newborns, suggesting reduced iron availability in these infants. Nonetheless, the differences were only found at birth, when lower levels were verified in the LPT newborns (p<0.001). These differences between the groups cannot be considered alone because serum iron is a less accurate indicator of iron reserves and may be influenced by several factors, such as stress, inflammatory processes, the release of erythrocytes by lysis and the mobilization of iron storage, among others (18).
In addition to their concentrations being within normal limits at two months, in LPT newborns, TIBC evolution after birth was within normal limits, according to the reference values of 250 to 390 µg/dl (19). The highest levels reached at one month post-T may reflect the beginning of an iron deficiency, which could have been the result of the higher growth rates observed in this period and a possible insufficient supply of iron.
TfSat is not a specific measure for determining iron reserves; however, levels above 20 to 25% exclude iron deficiency (20). TfSat was higher than 20% at birth in both groups, although the LPT values were much lower than those of the term newborns. As mentioned, the beginning of an iron deficiency could also be indicated by the hematological parameters and iron content evolution over one month post-T, according to the corrected GA, for LPT newborns reaching minimal acceptable levels, along with the intense decrease in Hb and increase in reticulocytes beyond the elevation of the TIBC, reinforcing this hypothesis.
Ferritin is considered a reference marker of iron storage, but its concentrations are hardly affected by the depletion of iron stores (21), which might partly explain the similarity observed between the groups and the large standard deviation found. In our study, the values found at birth for both term and LPT newborns were similar to those described in a recent review published by Lorenz et al. (22). After birth, ferritin levels decreased until two months of age in both groups. This evolution further strengthens the hypothesis of iron deficiency during a phase when a ferritin increase was expected following physiological hemolysis and iron release from internal stores (16).
In LPT newborns, lower concentrations of iron markers were observed at corrected GAs of term and one month post-T than those in term newborns. These results reinforce the evidence supporting reduced iron reserves in this group of infants, due to both the absence of an adequate iron supply during the third gestational trimester and possibly insufficient postnatal intake (22).
Nevertheless, if we consider that brain development may be compromised by low iron stores involving ferritin levels lower than 35 µg/l (23), there would have been no risk of injury to the central nervous system in our study because the values obtained were higher than 35 µg/l, at least the time period studied.
Based on these data, a pattern of accelerated growth and differences between the LPT and the term newborns were observed along with a reduction in hematological values, which could have been influenced by prematurity.
The inability to extend the clinical follow-up and the adversities encountered during medical care, including family problems, constituted research restrictions. Another possible limitation of this study was the lower significance, based on the large standard deviation found and the study of iron markers other than ferritin, which did not exhibit significant differences in our results.
Despite maternal iron supplementation during prenatal care and the first two months after delivery, our results revealed reduced stores of iron during this period in the newborns, which could indicate chronic iron deficiency.
Considering exclusively breastfed LPT newborns, we recommend closely monitoring hematological parameters and iron content evolution during the first months of life.
ACKNOWLEDGMENTSWe thank Sergio Mikio Koyama for help with the statistical analysis and Dr. Thelma Suely Okay, Gilda Maria Bárbaro Del Negro, Regina Miyuki Yamagata and Patrícia Palmeira for help with preparing the samples and conducting the laboratory tests.
AUTHOR CONTRIBUTIONSYamada RT and Leone CR participated in all aspects of the study (design, data collection, data analysis and manuscript writing) and accept responsibility for the content of the manuscript.
No potential conflict of interest was reported.