To determine the molecules involved in extracellular matrix remodeling and to identify and quantify heparanase isoforms present in herniated and degenerative discs.
INTRODUCTION:Heparanase is an endo-beta-glucuronidase that specifically acts upon the heparan sulfate chains of proteoglycans. However, heparanase expression in degenerative intervertebral discs has not yet been evaluated. Notably, previous studies demonstrated a correlation between changes in the heparan sulfate proteoglycan pattern and the degenerative process associated with intervertebral discs.
METHODS:Twenty-nine samples of intervertebral degenerative discs, 23 samples of herniated discs and 12 samples of non-degenerative discs were analyzed. The expression of both heparanase isoforms (heparanase-1 and heparanase-2) was evaluated using immunohistochemistry and real-time RT-PCR analysis.
RESULTS:Heparanase-1 and heparanase-2 expression levels were significantly higher in the herniated and degenerative discs in comparison to the control tissues, suggesting a possible role of these proteins in the intervertebral degenerative process.
CONCLUSION:The overexpression of heparanase isoforms in the degenerative intervertebral discs and the herniated discs suggests a potential role of both proteins in the mediation of inflammatory processes and in extracellular matrix remodeling. The heparanase-2 isoform may be involved in normal metabolic processes, as evidenced by its higher expression in the control intervertebral discs relative to the expression of heparanase-1.
It has been suggested that the majority of back pain is caused by intervertebral disc degeneration (IVD), which is a condition that can lead to spinal stenosis, radiculopathy, disc herniation, and age-related histological alterations.1,2
IVD describes a complex set of biochemical changes that can progress with age and is associated with increased cigarette smoking, obesity, diabetes and other heredity factors.3–6 However, the biological and pathological processes that occur during disc degeneration remain poorly understood. Therefore, existing medical and surgical treatments are limited and provide unpredictable outcomes.7
During aging, small leucine-rich proteoglycans located in the inner and outer annulus fibrosus (AF) and nucleus pulposus (NP) are structurally modified. The concentrations of small proteoglycans such as biglycan have been shown to increase in all regions of the disc (the AF and NP regions), whereas the levels of decorin and collagen content tend to decrease.8,9–11
Notably, heparan sulfate (HS) is present within the tissue microenvironment and functions as a major constituent of the HS proteoglycans (HSPG) and as free HS chains that are involved in angiogenesis and osteogenesis.12
Heparanase-1 (HPA1), a mammalian endo-beta-glucuronidase, promotes the cleavage of HS and generates oligosaccharides that are able to activate angiogenesis and the activity of growth factors, cytokines and chemokines.13,14
Most studies investigating HPA1 have focused on its regulated expression at different stages of cancer progression. In bone tissue, HPA1 overexpression creates a complex phenotype that typically results in osteogenesis and increased bone mass.15 Gomes et al.16 provided evidence of a dramatic loss of HS in the chondro-osseous junction during endochondral bone formation processes, suggesting that HS inhibits osteogenesis. Notably, heparanase-2 (HPA2), an isoform of heparanase, does not exhibit enzymatic activity and its function remains unclear.17,18
The main objective of our study was to evaluate the expression of both heparanase isoforms (HPA1 and HPA2) in IVD by comparing patient samples of herniated discs with samples of non-degenerative discs (control group) to better understand the role of HPA1 and HPA2 in IVD and to identify any corresponding extracellular matrix (ECM) alterations.
METHODSPatients and tissuesThe present study analyzed 29 samples of intervertebral degenerative discs, 23 samples of herniated discs and 12 samples of non-degenerative discs; the latter samples were obtained from surgeries performed on patients characterized with an accidental fracture. It is important to note that patients from the control group did not report any previous lumbar pain. The samples were collected between February 2008 and January 2009. The intervertebral disc specimen samples were collected via resection of the annulus fibrosis and nucleus pulposus of 53 patients (aged 18 to 43 years) and 12 healthy donors (aged 21 to 35 years). This study conformed to the regulations of the Human Ethics Research Committee at Faculdade de Medicina do ABC number 262/2008.
Histological and clinical featuresAll patients were subjected to magnetic resonance imaging (MRI), and the degenerative disc samples were characterized using the Pfirrmann grading system.19 T2-weighted sagittal images were used to determine the grade of the disc degeneration (grade 1: normal; grade 5: advanced degeneration). Only the specimens classified according to the MRI evaluation as Pfirrmann grades 2, 3 and 4 were further analyzed. Lumbar intervertebral disc tissues (AF or NP) were collected during the surgical procedure from patients with degenerative lumbar discs (patient group) or from individuals who, because of an accident, fractured their intervertebral disc, which thereafter required surgical removal. Histological parameters (including cellularity, inflammatory infiltrates, neovascularization, cell death, mucous degeneration, calcification, granular changes and clefts or microfractures) were analyzed at two regions of the disc, the NP and AF regions, using a semi-quantitative assessment performed using ten high-power fields (400X) ((0) = absent; (+) = rarely present; (++) = present in intermediate amounts; and (+++) = abundantly present).
In the present study, the samples from the degenerative disc group and the herniated disc group were classified as Pfirrmann grades II, III and IV, whereas the discs from the control group were classified only as Pfirrmann grade I. We excluded discs with a Pfirrmann grade I.
Immunohistochemical stainingRepresentative lumbar intervertebral degenerative disc regions were chosen based on the results of a hematoxylin-eosin staining study of the corresponding tissue sections. Three-micrometer-thick sections of formalin-fixed paraffin-embedded tissues were deparaffinized and rehydrated. The primary heparanase isoform antibodies HPA1 C-20 and HPA2 C-17 (Santa Cruz, Biotechnology, CA, USA) were diluted 1∶100 and incubated overnight. A universal secondary biotinylated antibody (LSAB®, DakoCytomation®, Glostrup, Denmark) was applied for 30 minutes, and the slides were subsequently incubated with a peroxidase-labeled streptavidin complex (LSAB®, DakoCytomation, Glostrup, Denmark) for an additional 30 minutes. The sections were developed using 3,3′-diaminobenzidine as the chromogen for one minute and were subsequently counterstained with hematoxylin.
Digital quantificationThe slides were analyzed using a TS100 Nikon Eclipse® light microscope to identify the areas that best represented the immunostaining of HPA1 and HPA2 (hot spots). In each case, quantification of the degree of immunostaining was performed using a digital computer-assisted image analysis method.20 Photomicrographs (640×480 pixels) were obtained from non-coincident consecutive fields for each case at a magnification of 400X using a 4300 Nikon Coolpix® digital camera adjusted to the same parameters. The resulting images were analyzed using the image processing and analysis system ImageLab® (Softium Informática®, São Paulo, Brazil) adjusted to the micrometer scale (μm). The index of the positive percentage of labeled cells (IP), the index of the immunostaining intensity expression (ItE) and the index of expression (IE) were obtained as described by Matos.20
RNA extraction and cDNA synthesisThe total RNA was extracted from the lumbar disc tissue resections using an RNAspin Mini Kit (GE Healthcare, Germany) according to the manufacturer's instructions. RNA quantification was performed using an Agilent 2100 Bioanalyzer with an RNA 6000 Nano LabChip Kit (Agilent Technologies, Palo Alto, CA, USA), and the RNA integrity was analyzed via agarose gel electrophoresis to identify the 28S and 18S ribosomal rRNA. The first-strand cDNA was synthesized using 5 μg of total RNA, 500 ng of oligo (dT) and Superscript III reverse transcriptase (Invitrogen, Carlsbad, USA). The reaction was performed at 50°C for 60 minutes followed by a 15-minute incubation period at 70°C.
Quantitative RT-PCRQuantitative RT-PCR was performed using SYBR Green I according to the manufacturer's instructions (Applied Biosystems, Foster City, CA, USA) in conjunction with Rotor-Gene 6000 Series Software 1.7 (Corbett Research, USA). The relative expression levels were measured using an efficiency correction,21 which considers the differences in primer-pair amplification efficiencies between the target and reference genes and results in a more reliable estimation of the “real expression ratio” than the 2ΔΔCt method.22 Measurements of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and the ribosomal protein RPL13a were performed and served as controls. The ΔCT value describes the difference between the CT value (cycle threshold) of the target gene and that of the corresponding endogenous reference gene (e.g., housekeeping gene): ΔCT = CT (target gene) – CT (endogenous reference gene). The ΔΔCT value represents the difference between the average ΔCT value of the sample of interest and the average ΔCT value of the reference sample. The PCR amplifications were performed using the following primer sequences: HPA1 forward primer: 5'-TGGCAAGAAGGTCTGGTTAGGAGA-3'; HPA1 reverse primer: 5'-GCAAAGGTGTCGGATAGCAAGGG-3'; GAPDH forward primer: 5'-GGAGAAGGCTGGGGCTC-3′; HPA2 forward primer: 5'-CGCCTGTTAGACACACTCTCTGA-3'; HPA2 reverse primer: 5'-GTCACCACACCTTCAAGCCAA-3'; GAPDH reverse primer: 5'-GTCCTTCCACGATACCAAAG-3′; the ribosomal protein (RPL13a) forward primer 5'-TTGAGGACCTCTGTGTATTTGTCAA-3′; and the RPL13a reverse primer 5'-CCTGGAGGAGAAGAGGAAAGAGA-3′.
Statistical analysisStatistical analysis was performed using the SPSS 17.0 program for Windows (SPSS, Chicago, IL). The parametricity of variables was determined using the Kolmogorov-Smirnov test. The Chi-square test and the t-test were used for parametric analyses, whereas the Mann-Whitney and Kruskal-Wallis tests were used for non-parametric analyses. The level of statistical significance adopted for all analyses was P<0.05.
RESULTSThe average age of patients in this study with lumbar disc degeneration, the average age of patients with herniated discs and the average age of controls was 33.4±7.03, 34.1±6.61 and 29.5±6.26 years, respectively. There were no statistically significant differences among these groups with regard to the average age (P = 0.199, Kruskal-Wallis test).
Of the 53 intervertebral lumbar discs that were resected from patients and subsequently evaluated, there were 29 degenerative discs (54.7%) and 24 herniated discs (45.3%). There were statistically significant differences between the histological and clinical features observed for the herniated and control discs, including cellularity, neovascularization, mucous degeneration, calcification, granular changes, clefts or microfracture formation, and the presence or absence of lumbar disc herniation (Table 1). However, no statistically significant differences were observed regarding inflammatory infiltrates and cell death upon comparison of the herniated disc group and the control group (Table 1).
Clinical features and histological parameters in herniated and control discs.
| Group | |||
|---|---|---|---|
| Herniated(n = 23) | Control(n = 12) | P | |
| Cellularity | |||
| (+) | 4 (17.4) | 0 (0.0) | 0.042 * |
| (++) | 14 (60.9) | 12 (100.0) | |
| (+++) | 5 (21.7) | 0 (0.0) | |
| Inflammatory infiltrates | |||
| 0 | 17 (73.9) | 12 (100.0) | 0.151 |
| (+) | 4 (17.4) | 0 (0.0) | |
| (++) | 2 (8.7) | 0 (0.0) | |
| Neovascularozation | |||
| 0 | 13 (56.5) | 12 (100.0) | 0.026 * |
| (+) | 6 (26.1) | 0 (0.0) | |
| (++) | 4 (17.4) | 0 (0.0) | |
| Cell Death | |||
| (+) | 3 (13.0) | 0 (0.0) | 0.251 |
| (++) | 12 (52.2) | 5 (41.7) | |
| (+++) | 8 (34.8) | 7 (58.3) | |
| Mucous degeneration | |||
| (+) | 0 (0.0) | 6 (50.0) | <0.001 * |
| (++) | 12 (52.2) | 5 (41.7) | |
| (+++) | 11 (47.8) | 1 (8.3) | |
| Calcification | |||
| 0 | 11 (47.8) | 11 (91.7) | 0.035 * |
| (+) | 7 (30.4) | 1 (8.3) | |
| (++) | 5 (21.7) | 0 (0.0) | |
| Granular changes | |||
| 0 | 5 (21.7) | 3 (25.0) | 0.027 * |
| (+) | 7 (30.4) | 9 (75.0) | |
| (++) | 8 (34.8) | 0 (0.0) | |
| (+++) | 3 (13.0) | 0 (0.0) | |
| Clefts or microfractures | |||
| 0 | 4 (17.4) | 0 (0.0) | 0.034 * |
| (+) | 7 (30.4) | 0 (0.0) | |
| (++) | 10 (43.5) | 9 (75.0) | |
| (+++) | 2 (8.7) | 3 (25.0) | |
Percentage (%); * Statistical significance (Chi-square test).
Similarly, cellularity, mucous degeneration, calcification processes, granular changes, clefts or microfracture formation were statistically different upon comparison of the degenerative discs with the control discs (Table 2), whereas no statistically significant differences were observed between these two groups for the clinical features and histological parameters including inflammatory infiltrates, neovascularization and cell death (Table 2).
Clinical features and histological parameters in degenerative and control discs.
| Group | |||
|---|---|---|---|
| Degenerative(n = 29) | Control(n = 12) | P | |
| Cellularity | |||
| (+) | 10 (34.5) | 0 (0.0) | 0.004 * |
| (++) | 13 (44.8) | 12 (100.0) | |
| (+++) | 6 (20.7) | 0 (0.0) | |
| Inflammatory infiltrates | |||
| 0 | 21 (72.4) | 12 (100.0) | 0.128 |
| (+) | 7 (24.1) | 0 (0.0) | |
| (++) | 1 (3.4) | 0 (0.0) | |
| Neovascularozation | |||
| 0 | 19 (65.5) | 12 (100.0) | 0.065 |
| (+) | 6 (20.7) | 0 (0.0) | |
| (++) | 4 (13.8) | 0 (0.0) | |
| Cell Death | |||
| (+) | 3 (10.3) | 0 (0.0) | 0.131 |
| (++) | 18 (62.1) | 5 (41.7) | |
| (+++) | 8 (27.6) | 7 (58.3) | |
| Mucous degeneration | |||
| (+) | 1 (3.4) | 6 (50.0) | 0.001 * |
| (++) | 18 (62.1) | 5 (41.7) | |
| (+++) | 10 (34.5) | 1 (8.3) | |
| Calcification | |||
| 0 | 13 (44.8) | 11 (91.7) | 0.019 * |
| (+) | 8 (27.6) | 1 (8.3) | |
| (++) | 8 (27.6) | 0 (0.0) | |
| Granular changes | |||
| 0 | 6 (20.7) | 3 (25.0) | 0.012 * |
| (+) | 8 (27.6) | 9 (75.0) | |
| (++) | 10 (34.5) | 0 (0.0) | |
| (+++) | 5 (17.2) | 0 (0.0) | |
| Clefts or microfractures | |||
| 0 | 6 (20.7) | 0 (0.0) | <0.0001 * |
| (+) | 15 (51.7) | 0 (0.0) | |
| (++) | 5 (17.2) | 9 (75.0) | |
| (+++) | 3 (10.3) | 3 (25.0) | |
Percentage (%); * Statistical significance (Chi-square test)
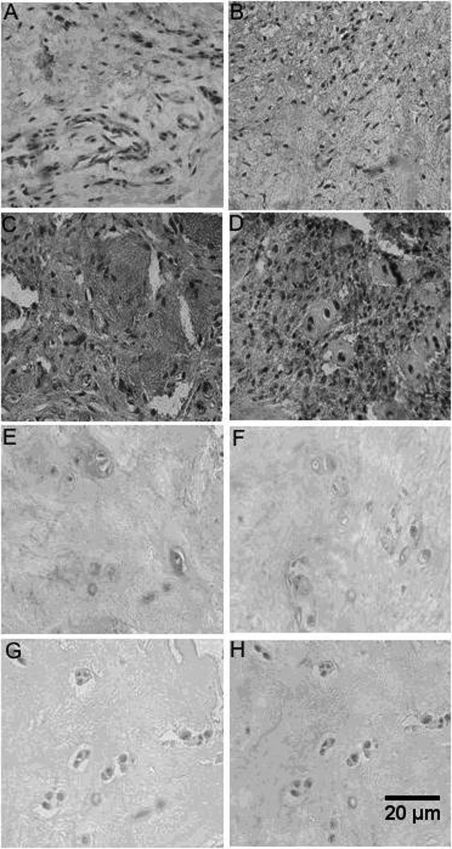
HPA1 and HPA2 were identified using immunohistochemistry in the NP and AF regions of the control lumbar intervertebral discs (Figure 1). As demonstrated in Figure 1, the expression of both protein isoforms (HPA1 and HPA2) was higher in the degenerative discs than in the control discs.
Fibrous annulus and pulposus nucleus HPA1 and HPA2 immunohostochemistry. A, Fibrous annulus control disc HPA1 C20 Santa Cruz primary antibody; B, Fibrous annulus control disc HPA2 C17 primary antibody; C, Fibrous annulus degenerative disc HPA1 C20 Santa Cruz primary antibody; D, Fibrous annulus degenerative disc HPA2 C17 Santa Cruz primary antibody; E, Pulposus nucleus control disc HPA1 C20 Santa Cruz primary antibody; F, Pulposus nucleus control disc HPA2 C17 Santa Cruz primary antibody; G, Pulposus nucleus degenerative disc HPA1 C20 Santa Cruz primary antibody; H, Pulposus nucleus degenerative disc HPA2 C17 Santa Cruz primary antibody. Magnification: 400X.
Figures 1A and 1B demonstrate the negative immunoreactivity of HPA1 and HPA2, respectively. Although many fibroblasts were observed in the control AF, no vessels were observed. Moreover, evidence of neovascularization was observed in Figure 1C (degenerative AF). Although there was almost no HPA1 immunoreactivity observed in the fibroblasts, intense labeling could be observed in the extracellular matrix. Conversely, intense HPA2 immunoreactivity was observed in the inflammatory infiltrate of the AF of the degenerative discs, whereas low levels of labeling were observed in the extracellular matrix (Figure 1D).
A lack of immunoreactivity for HPA1 and HPA2 were observed in the chondrocytes and the extracellular matrix of the control NP (Figures 1E and 1F), respectively. Strong positive immunoreactivities for HPA1 and HPA2 were observed in the NP region of the degenerative lumbar discs (Figures 1G and 1H, respectively), which was also characterized by hypercellularity.
The expression index (IE) of HPA1 was significantly higher in the herniated (76.78±29.19 ou/μm2) and degenerative lumbar intervertebral discs (83.59±33.20 ou/μm2) when compared with the control intervertebral discs (19.46±5.23 ou/μm2, P<0.001, Mann-Whitney test, Figure 2). Similarly, the expression of the HPA2 isoform was significantly higher in the herniated (82.67±29.72 ou/μm2) and degenerative discs (81.37±29.04 ou/μm2) when compared with the control discs (28.25±10.19 ou/μm2, P<0.001, Mann-Whitney test).
Analysis of HPA1 and HPA2 immunohistochemical results. The values indicate the expression index (IE) obtained from digital quantification after immunohistochemical reactions using specific HPA1 C20 and HPA2 C17 antibodies (Santa Cruz, Biotechnology, CA, USA) as described in the Methods. **P<0.001, Mann-Whitney Test, in comparison with the control IE values.
None of the clinical or histological parameters reported in Tables 2 and 3 for the herniated discs (n = 24 patients) and degenerative discs (n = 29 patients, data not shown) were statistically different.
Although both IP and IE indices indicated higher expression levels in the herniated and degenerative discs in comparison to the control group, no statistically significant differences were observed between the HPA1 and HPA2 immunoexpression intensity index (ItE) (data not shown).
There was a higher expression of the HPA2 isoform (28.25±10.19 ou/μm2) than the HPA1 isoform (19.46±5.23 ou/μm2, P = 0.028, Mann-Whitney test) in the control discs.
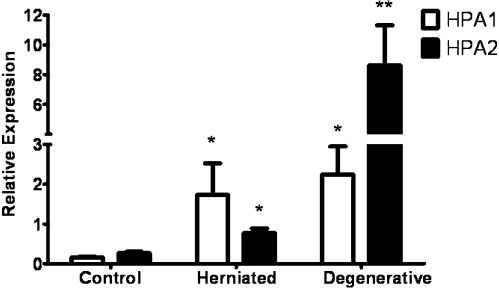
The expression of the heparanase isoforms was also investigated using quantitative RT-PCR analysis. The HPA1 and HPA2 mRNAs were more highly expressed in the herniated and degenerative intervertebral discs than in the control intervertebral discs (Figure 3).
Quantitative HPA1 and HPA2 expression. Statistical analyses were performed using the Student t-test. *P<0.05, comparison of the control group to the herniated and degenerative disc groups; **P<0.001, statistically significant difference between HPA2 expression in the herniated and degenerative groups.
The quantitative RT-PCR analysis revealed significantly higher HPA1 expression in the herniated intervertebral discs (1.73±0.80) when compared with the control intervertebral discs (0.16±0.02, P<0.05, Student's t-test, Figure 3).
Similar results were obtained for the HPA2 qRT-PCR, which indicated an increased expression of HPA2 in the herniated discs in comparison to the control discs, with values of 0.77±0.12 and 0.27±0.03, respectively (P<0.05, Student's t-test, Figure 3).
The HPA1 expression was significantly higher in the degenerative discs (2.25±0.70) than in the control group (0.16±0.02, P<0.05, Student's t-test). In addition, these results indicated that HPA2 was overexpressed in the degenerative discs in comparison to the control discs, with values of 8.62±2.69 and 0.27±0.03, respectively (P<0.001, Student's t-test, Figure 3).
The immunohistochemistry and qRT-PCR analyses confirmed that the HPA2 isoform was up-regulated by approximately 60–70% in the control discs in comparison to the HPA1 isoform and also that the herniated and degenerative discs expressed significantly higher amounts of both heparanase isoforms when compared with the control discs (i.e., discs not affected by intervertebral disease).
As shown in Figure 3, the quantitative RT-PCR demonstrated a statistically significant difference in the expression of HPA1 and HPA2 in the herniated and degenerative discs. HPA1 and HPA2 mRNA levels were higher in the degenerative discs.
DISCUSSIONThe observation of different rates of lumbar disc degeneration has suggested the existence of distinct pathways of degenerative intervertebral disc disease. One such pathway for disc degeneration involves an inflammatory response associated with an injury or other mode of mechanical stress, whereas aging represents a normal progression of the degenerative process with observable cellular and molecular changes in the ECM.23 It is important to note that the present study evaluated a group of young individuals within a fairly finite age range, which necessarily precludes the ability to draw conclusions based on age-dependent and occupation-dependent relationships.
This study demonstrates that the expression levels of both heparanase isoforms (HPA1 and HPA2) are significantly higher in degenerative intervertebral AF and NP tissues when compared with control tissues, suggesting a possible role of HPA1 and HPA2 in the pathophysiology of this disease. It is important to note that the chondrocytes in the degenerative NP discs studied herein were intensely immunoreactive for both HPA1 and HPA2.
The histological analysis demonstrated a significant increase in the neovascularization of the herniated discs in comparison to the control discs. In addition, the immunohistochemical analysis of the AF region clearly demonstrated that HPA2 was significantly overexpressed in the inflammatory infiltrate, whereas the HPA1 immunoreaction was more prominent in the extracellular matrix.
Moreover, it is known that structural changes to the cartilage endplate reduce the nutrient supply, causing continuous adaptive tissue changes that may stimulate intervertebral degeneration and subsequent modifications in the heparan sulfate proteoglycan distribution within the basal membrane, not unlike what happens to perlecan. Perlecan has the ability to sequester growth factors and organize a diverse range of matrix components such as those in the fetal spine.24,25 Such alterations of the heparan sulfate proteoglycan structure in the extracellular matrix may be explained by HPA1 overexpression, which degrades heparan sulfate chains that primarily regulate the biological function of proteoglycans.
It has been proposed that heparanase up-regulation may be a novel therapeutic approach for the prevention and treatment of osteoporosis because heparanase facilitates cartilage replacement with bone at the chondro-osseous junction by removing the heparan sulfate component of proteoglycans (e.g. perlecan/HSPG2) that would otherwise prevent osteogenic cells from remodeling the hypertrophic cartilage.26
It is also interesting to note that HPA2 was more highly expressed than HPA1 in the control discs and in the degenerated discs. This result suggests that HPA2 could be involved in the normal metabolism of tissue because it has no enzymatic activity. Therefore, in the control intervertebral discs, extracellular remodeling would be uncommon, and heparan sulfate degradation would be most likely controlled by HPA1. However, in the degenerative and herniated discs, HPA1 was overexpressed and may have subsequently induced the formation of heparan sulfate oligosaccharides. These specific oligosaccharides are known to be involved with neovascularization, cell proliferation and cell migration.14 Brown et al.26 demonstrated that heparanase expression and activity influences chondrogenic and osteogenic processes during endochondral bone formation. In addition, a recent study proposed that enhanced osteoclastogenesis is promoted by high levels of heparanase expressed in the cells, resulting in a dramatic increase in bone reabsorption in vitro.27 Moreover, it has been found that syndecan-1 mediates distal cross-talk with bone that enhances osteoclastogenesis,27 and it has been reported that high levels of HPA1 result in enhanced shedding of syndecan-1.28
HPA1 and HPA2 overexpression was confirmed by the analysis of protein and mRNA levels using immunohistochemistry and quantitative RT-PCR, respectively. These data validated once more that qRT-PCR represents a precise methodology for differentiating between HPA1 and HPA2 expression in herniated, degenerative and control lumbar intervertebral discs. These tissue analyses illustrated the different ECM components present in these groups of discs, which reflects an intense remodeling process in the degenerative discs and a less intense display of alterations in the herniated discs, in addition to a fairly stable equilibrium state and minimal remodeling process in the control discs.
The increased HPA1 expression in the herniated intervertebral discs may indirectly facilitate endothelial cell migration and proliferation.29 In addition, the HPA2 isoform may be involved with normal metabolic processes as suggested by the overexpression observed in the control intervertebral discs compared with the expression of the HPA1 isoform.
The results also led us to propose possible roles for both heparanase isoforms in the calcification process of intervertebral discs during herniation development.
For the first time, we report that degenerative intervertebral discs present a significant increase in the level of heparanase expression, which suggests that heparan sulfate proteoglycan alterations and extracellular matrix remodeling changes are important events that may lead to a better understanding of the molecular mechanisms of this disease.