The purpose of this study was to analyze the effects of soybean extracts obtained using different extraction methods on the skin of female rats.
METHOD:A total of 64 female Sprague-Dawley rats were divided into 8 equal groups. Various extracts were administered to the female rats by oral gavage for one month. The groups comprised carboxymethyl cellulose-free control, carboxymethyl cellulose-plus control, 100-mg/kg n-hexane extract, 200-mg/kg n-hexane extract, 100-mg/kg ethyl acetate extract, 200-mg/kg ethyl acetate extract, 100-mg/kg ethanol extract and 200-mg/kg ethanol extract groups. The thickness of the collagen layer and the number of estrogen receptor-positive cells were evaluated.
RESULTS:All the extract-treated groups showed a statistically significant decrease in the number of estrogen receptor-positive cells compared with the control groups. Regarding the thickness of the collagen layer, only the 200-mg/kg ethyl acetate extract-treated group showed a significant increase compared with the control groups (p<0.05).
CONCLUSIONS:Our data suggest that oral intake of three different total soybean extracts might have positive estrogenic effects on the skin and that only a high-dose ethyl acetate extract can increase the expression of collagen, which may prove to be beneficial for postmenopausal facial skin.
The soybean Glycine max (L.) Merr. (Leguminosae) is a species of legume that is native to East Asia and is considered a very important plant because of its phytochemical content. Glycine max L. is widely used in the food industry as a supplement and is included in the formulations of many drugs. Recently, the use of soybeans has been extensively studied for the prevention and treatment of various chronic diseases (1–3).
The conclusions that were reached by most of the above-mentioned studies indicated that the positive effects of soybean products can be attributed to the presence of isoflavones, a class of organic compounds that are produced almost exclusively by members of the Fabaceae/Leguminosae (bean) family (4). The most important soy isoflavones are genistein, daidzein and glycitein. Isoflavones are phytoestrogenic compounds that are structurally similar to estrogenic steroids.
In this study, the positive effects of soy isoflavones on the skin were investigated. Soy isoflavones are considered to have beneficial effects on the skin via mechanisms such as the prevention of lipid oxidation, stimulation of fibroblast proliferation, reduction of collagen degradation and inhibition of 5α-reductase and they have been widely used as ingredients in cosmetics. Moreover, cosmetics containing isoflavones are reported to retard skin aging (5).
We hypothesized that if soy isoflavones could be shown to have positive estrogenic effects on the skin and collagen layer, then they could be used in the management of skin and hair diseases that are caused by estrogen insufficiency.
Soybeans have been used in Asia as a crop rotation material; however, soy products were not served as food until precipitation and fermentation techniques were developed. The Chinese did not eat soybeans because they were thought to contain “harmful substances” that produced serious gastric distress.
Although genistein and daidzein may act on the estrogen receptor, other soy products, such as soy proteins, may have some biological effects and interfere with the effect of isoflavones. This fact notwithstanding, the processing of soy extract is easier and cheaper than obtaining purified daidzein and genistein and soy extract is more stable than these compounds (6). For these reasons, we chose to use soy extracts in this study.
Here, we investigated the in vivo effects of various soybean extracts, in which Glycine max L. phytoestrogens were previously detected in phytochemical studies, on the skin of rats.
MATERIALS AND METHODSGlycine max plants that were naturally growing in Turkey were collected under the appropriate conditions and were dried for use in the experiments. N-hexane, ethyl acetate and ethanol extracts were prepared from the collected study material for the purpose of analyzing their biological activity. Preliminary tests were conducted using the prepared extracts. Subsequently, a method that was found to be effective for quantifying isoflavones in the extracts was performed using high-performance liquid chromatography (HPLC).
The experiments were performed at the Experimental Animal Laboratory of Afyon Kocatepe University, Turkey. The study protocol was approved by the local ethics committee.
Animals and treatmentsSixty-four 2-month-old female Sprague-Dawley rats weighing 180-220 g were divided into eight groups with eight rats per group. During the experimental period, the animals were maintained under standard conditions: a 12-hour light-dark cycle with food and water provided ad libitum, a temperature of 21°C and>50% humidity. During the study period, the shelter were cleaned every two days and the food and water were refreshed. The specific soy extracts for each group were administered by oral gavage every day for one month. The daily dosage was adjusted to 1 ml and contained soybean extracts at a concentration of 100 or 200 mg/kg. At the end of the study, the animals were euthanized by cervical dislocation under general anesthesia (21.1 mg/kg ketamine and 4.2 mg/kg xylazine) and skin samples were collected.
Experimental groupsGroup A was the carboxymethyl cellulose (CMC)-free control group (no substance/solvent-applied group). Group B was the CMC-plus control group (0.5% CMC-treated group; this CMC solution was used to dilute the extracts for administration to the experimental animals). Group C included the rats that received the n-hexane extract at a dose of 100 mg/kg. Group D included the rats that received the n-hexane extract at a dose of 200 mg/kg. Group E included the rats that received the ethyl acetate extract at a dose of 100 mg/kg. Group F included the rats that received the ethyl acetate extract at a dose of 200 mg/kg. Group G included the rats that received the ethanol extract at a dose of 100 mg/kg. Group H included the rats that received the ethanol extract at a dose of 200 mg/kg.
Histological and immunohistochemical evaluationFor the histological analysis, tissue samples (skin) were collected from the backs of the rats. The samples were fixed using a 10% formalin solution and embedded in paraffin and 4-µm-thick sections were then cut. After deparaffinization, the sections were stained with hematoxylin–eosin (HE) or Masson's trichrome and immunohistochemical staining of the ER (Dako, clone EP1, ready to use) was performed using a Dako Autostainer 48 Link (Dako, Denmark). The number of estrogen receptor-positive cells in the dermis was counted in 10 high-power fields. Masson's trichrome (VMS chemicals, Kavi Nagar, Ghaziabad, India) staining was performed according to the manufacturer's instructions. The thickness of the collagen layer in each rat skin sample was measured using Olympus image analysis software (DP21) under a light microscope. The mean values of the results obtained for the groups were compared and statistically evaluated.
Preparation of soybean extractsThe methods for the preparation and implementation of the extracts are described below. The collected soybeans were gradually extracted.
Preparation of the n-hexane extractSoybeans (25 g) were extracted 2 times at room temperature with shaking for 48 hours using 500 ml of n-hexane. The combined n-hexane extracts were dried in a vacuum desiccator under reduced pressure and concentrated using a rotavapor at 40°C.
Preparation of the ethyl acetate extractSoybeans (25 g) were extracted 2 times at room temperature with magnetic stirring for 48 hours using 500 ml of ethyl acetate. The combined ethyl acetate extracts were dried in a vacuum desiccator under reduced pressure and concentrated using a rotavapor at 40°C.
Preparation of ethanol extractsSoybeans (25 g) were extracted 2 times at room temperature with shaking for 48 hours using 500 ml of 70% ethanol containing 0.1% acetic acid. The combined ethanol extracts were dried in a vacuum desiccator under reduced pressure and concentrated using a rotavapor at 40°C (7).
HPLC analysisThe samples were centrifuged for 10 minutes at 13,500 rpm at 110°C (Eppendorf centrifuge mod. 5417R). After centrifugation, 100 µl of the supernatant was transferred to an autosampler (the injection volume was 10 µl). Isoflavone analysis was performed using an ODS c-18 column (YMC-Pack ODS-AM; S-5 mm, 120A; 250×4.6 mm I.D.).
The mobile phase (solvent A) was a solution of acetonitrile and 0.1% acetic acid and solvent B was a solution of water and 0.1% acetic acid. The initial gradient was 20% of solvent A for the first 20 minutes followed by 100% for 5 minutes and then 20% for the last 15 minutes. The effluent was monitored at 260 nm. The elution of each sample was completed in 40 minutes. Standard solutions of daidzin, daidzein, genistin and genistein (SIGMA) were prepared at a concentration of 0.0125 mg/ml (7).
Statistical analysisStatistical analyses were performed using SPSS software (Statistical Package for the Social Sciences, version 20.0; SPSS Inc., Chicago, IL, USA). All the data are expressed as the mean values±SD. The Kruskal-Wallis test was used for multiple statistical comparisons. Because there are 10 paired groups to compare, the total error was calculated as 0.05/10 and p<0.005 was considered to be statistically significant for evaluation using the post hoc multiple comparison test. The Mann-Whitney U test was used for two-group comparisons. The correlation test was used for the analysis of between-group correlations. P<0.05 was considered to be statistically significant.
RESULTSIn the n-hexane extract, the average total isoflavone concentration was 27 mg/25 g, comprising 40% daidzin, 56% genistin, 2% daidzein and 2% genistein. In the ethyl acetate extract, the average total isoflavone concentration was 48 mg/25 g, comprising 37% daidzin, 58% genistin, 2% daidzein and 3% genistein. In the ethanol extract, the average total isoflavone concentration was 52 mg/25 g, comprising 36% daidzin, 59% genistin, 2% daidzein and 3% genistein.
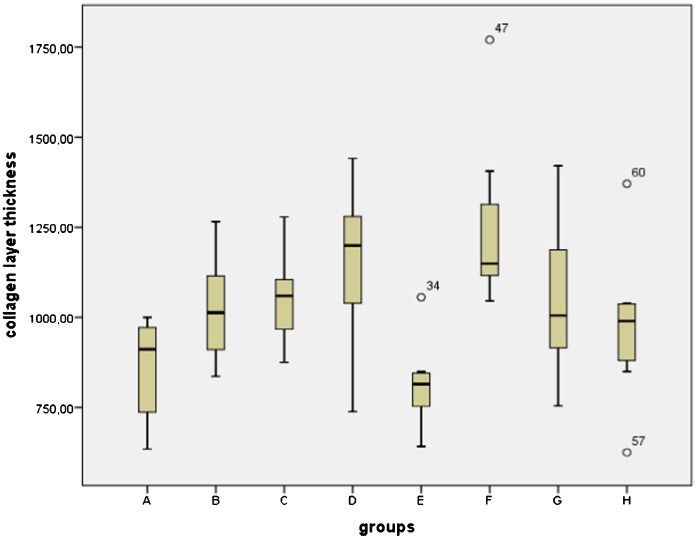
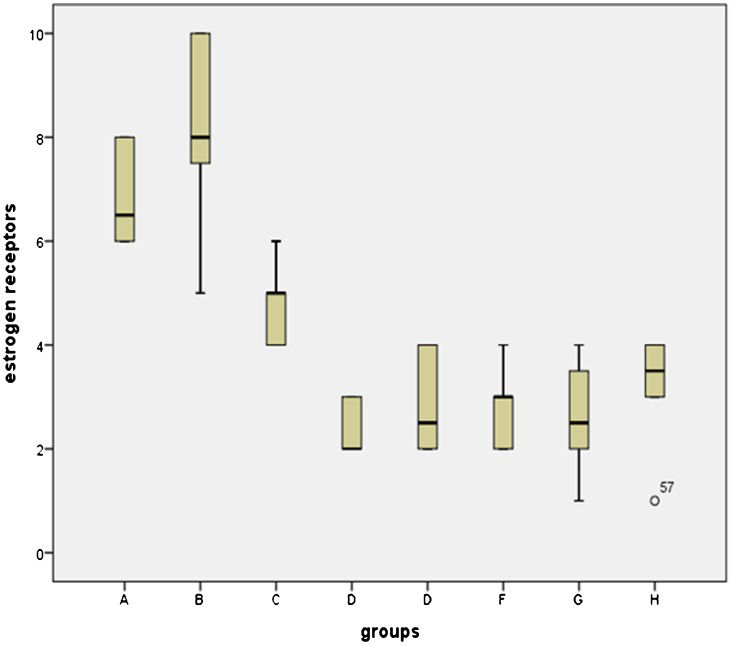
The mean values of collagen layer thickness and number of estrogen receptor-positive cells in the different soybean extract-treated groups are shown in Table1. The collagen layer thicknesses and the numbers of estrogen receptor-positive cells are presented in box plots (Figures1 and 2).
Mean values of the collagen layer thickness and the number of estrogen receptor-positive cells in the skin of animals in the different groups.
| Groups | Collagen thickness (µm) Mean ± SD | Number of estrogen receptors (per 10 high-power fields) Mean ± SD |
|---|---|---|
| Group A | 864.32±140.04 | 6.87±0.99 |
| Group B | 1264.06±510.95 | 8.25±1.75 |
| Group C | 1052.00±123.63 | 4.75±0.70 |
| Group D | 1152.35±220.06 | 2.37±0.51 |
| Group E | 798.91±84.48 | 2.87±0.99 |
| Group F | 1154.93±118.05 | 2.75±0.70 |
| Group G | 1049.12±213.20 | 2.62±1.06 |
| Group H | 976.53±209.93 | 3.25±1.03 |
By comparing the median values for the groups, a statistically significant reduction in the number of estrogen receptor-positive cells and an increase in the collagen layer thickness were observed (p<0.05). The collagen layer thickness and the number of estrogen receptor-positive cells of Group A and Group B were not significantly different. The thickness of the collagen layer of the rats in Group F was significantly increased compared with that of the rats in Group A (p = 0.001). There were no significant differences in the collagen layer thicknesses of the other groups compared to group A or group B. There were also no differences among the groups treated with different doses of soybean extract when they were compared to each other. There were statistically significant differences between group A and all the other groups in terms of the number of estrogen receptor-positive cells (except for Group B). All the p-values were 0.001 (Figure3). The number of estrogen receptor-positive cells in group D was significantly reduced compared with that of group C (p = 0.001). Therefore, increasing the dose of the n-hexane extract increased its effects.
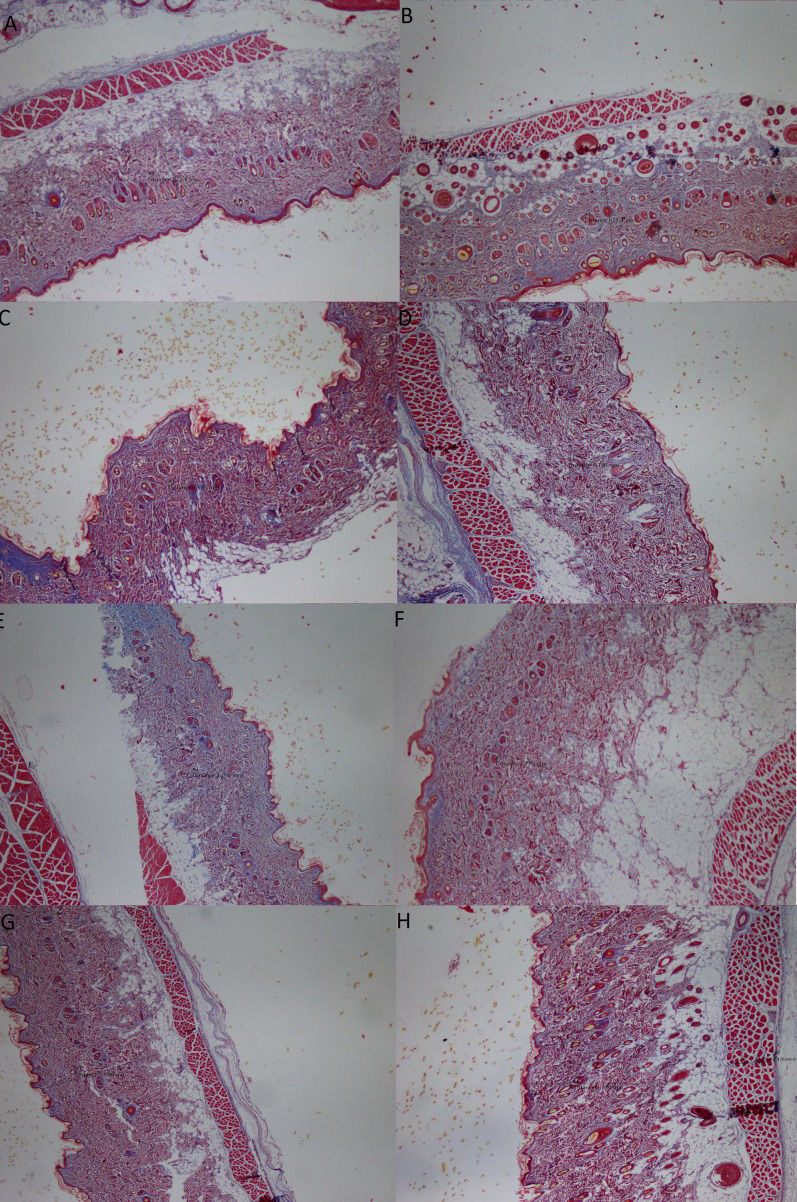
Histological evaluation of Masson's trichrome-stained sections of skin biopsies from the CMC-free group (A), the 0.5% CMC-plus group (B), the group treated with the n-hexane extract at a dose of 100 mg/kg (C), the group treated with the n-hexane extract at a dose of 200 mg/kg (D), the group treated with the ethyl acetate extract at a dose of 100 mg/kg (E), the group treated with the ethyl acetate extract at a dose of 200 mg/kg (F), the group treated with the ethanol extract at a dose of 100 mg/kg (G), and the group treated with the ethanol extract at a dose of 200 mg/kg (H).
There were no statistically significant differences between group E and group F and group H (Figure4). We did not observe any correlation between the collagen layer thickness and the number of estrogen receptor-positive cells.
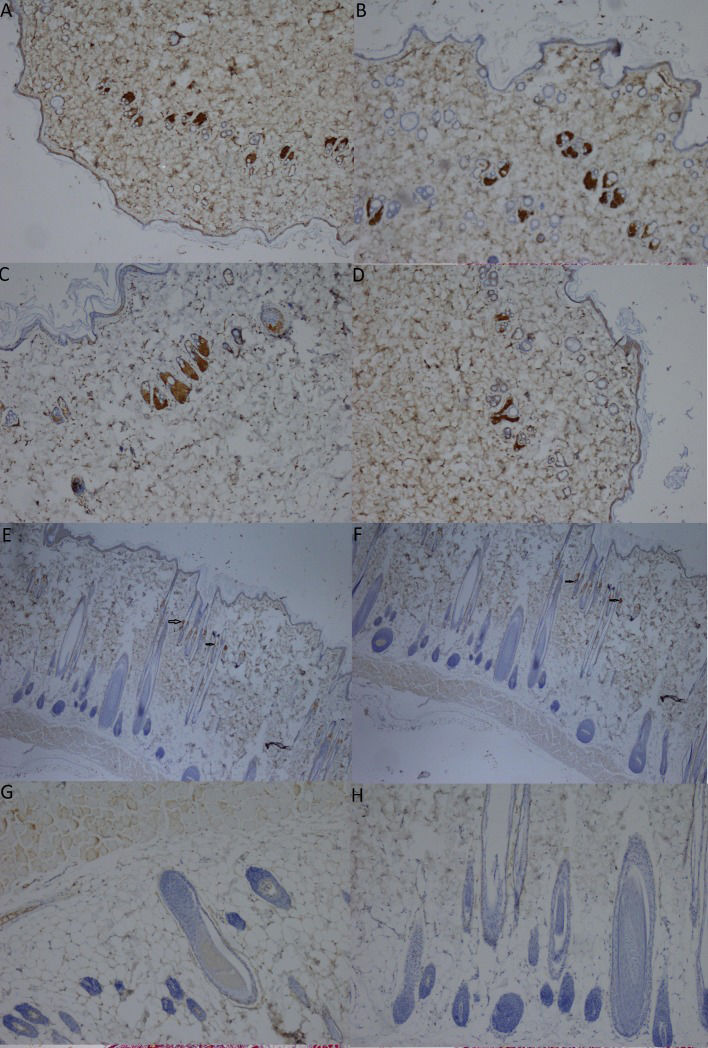
Immunohistochemical staining of the ER (estrogen receptor) in skin biopsies from the CMC-free group (A), the 0.5% CMC-plus group (B), the group treated with the n-hexane extract at a dose of 100 mg/kg (C), the group treated with the n-hexane extract at a dose of 200 mg/kg (D), the group treated with the ethyl acetate extract at a dose of 100 mg/kg (E), the group treated with the ethyl acetate extract at a dose of 200 mg/kg (F), the group treated with the ethanol extract at a dose of 100 mg/kg (G), and the group treated with the ethanol extract at a dose of 200 mg/kg (H) (magnification, 40X). The arrows indicate positively stained cells in the dermal layer.
Although systemic menopausal hormone therapy has been used for many years, recent trials have reported a significantly increased risk of breast cancer and other pathologies with this treatment (8). This has lead to a reconsideration of the risks and benefits of systemic menopausal hormone therapy. For this reason, high doses of systemic menopausal hormones cannot be recommended to treat skin aging (9).
The soybean Glycine max (L.) contains vegetable proteins, oligosaccharides, dietary fiber, vitamins, isoflavones and minerals. Earlier studies have demonstrated the cholesterol-lowering, skin-protective, antitumor, antidiabetic and antioxidative potentials of soybeans (10). The soybean plant is rich in isoflavones such as genistein and daidzein, which are structurally similar to estrogenic steroids. Soy isoflavones are also utilized in estrogen-replacement therapy for postmenopausal women.
Soy isoflavones alone or in combination with other agents have many beneficial effects on the appearance and structure of skin (5),. Accorsi-Neto et al. reported a thickened epidermis, increased collagen and elastin fiber content within the dermis, decreased thickness papillary dermis and a significantly increased amount of dermal blood vessels in 30 women with menopause who ate a soy isoflavone-rich diet for 6 months (13).
However, Patriarca et al. showed that the topical use of 0.01% estradiol and 4% genistein on the facial skin of postmenopausal women for 24 weeks led to an increase in the hyaluronic acid concentration in the dermis with no systemic consequences. However, the effect of estradiol has been demonstrated to be greater than that of genistein. Patriarca et al. attributed this finding to a different conclusion: the overall effect of isoflavones arises from both their effects on the estrogen receptor and their antioxidant effects, which, together, would protect against both intrinsic chronological cutaneous aging and extrinsic aging, which is mainly caused by ultraviolet radiation (14).
Phytoestrogens may act as both estrogen agonists and estrogen antagonists. They exert estrogenic effects on estrogen agonists. As antagonists, they suppress the effects of natural estrogen by binding to the estrogen receptor (15,16). In vitro and in vivo studies have shown that genistein demonstrates weak estrogenic and antiestrogenic properties (17). Among the phytoestrogens, genistein, which has the strongest effects, has a molecular structure that is very similar to that of estradiol. The intramolecular distances of the hydroxyl groups at both ends of the molecules are nearly identical, which indicates that they can easily bind to estrogen receptors. In this study, the number of estrogen receptor-positive cells was significantly decreased in the skin of rats that received soy isoflavones obtained using three different extraction methods. The reason for this decrease might be that the soy isoflavones bound to the estrogen receptors in the skin.
Carbonel et al. revealed that a high dose of isoflavone-rich soy extract (120 mg/kg per day) may have positive effects on the vaginal structures of ovariectomized rats; however, the effect of this treatment on vaginal thickness is weaker than that of estrogen treatment. In addition, soy extract may not block the effects of estrogen on vaginal tissues (6). Accordingly, Diel et al. demonstrated that oral administration of genistein at doses from 25 up to 100 mg/kg per day weakly increased the uterine weight and when ovariectomized animals were treated for 3 days with 100 mg/kg genistein per day, a significant increase in the uterine and the vaginal epithelial thickness was observed (18).
Over the last few decades, selective estrogen receptor modulators (SERMs) such as raloxifene have been developed. These modulators achieve the same beneficial effects as estrogen while minimizing harmful side effects in target tissues via specific estrogen-receptor interactions (19). Polito et al. revealed that both estradiol and genistein are more effective than raloxifene for improving skin damage related to decreased estrogen conditions (20). Recently, it was reported that sustained exposure to estrogen markedly delayed wound re-epithelialization in mice. In contrast, systemic treatment with genistein has been found to accelerate wound healing in ovariectomized rats (21). Polito et al. revealed that, surprisingly, the lowest genistein dose was more effective for restoring skin properties than raloxifene or estradiol. This finding was further supported by the finding that genistein can increase cell migration, inflammation, provisional matrix synthesis, collagen deposition, angiogenesis and re-epithelialization, all of which are crucial processes in skin repair. Interestingly, the experimental data obtained by Polito et al. also indicated that the systemic administration of genistein in the same dose range as that used in postmenopausal women had positive effects on ovariectomy-induced skin changes (22–28). Genistein has recently been accepted as the ideal natural SERM that might play a preventative role in the age-related skin changes accelerated by menopause without causing harmful estrogenic side effects in reproductive tissues (29). Currently, genistein-containing cosmetic creams are used to improve skin dryness and wrinkles related to estrogen deprivation (30). The efficacy and safety of genistein in a low-estrogen environment were demonstrated in experimental and clinical studies (22–28), (31–33).
The present study was undertaken to investigate the effect of soybean extracts that were obtained using three different extraction methods on the thickness of the collagen layer and on the abundance of estrogen receptor-positive cells in the skin of rats with a normal estrogenic status. In our study, under normal estrogenic conditions, the collagen layer thickness of the rats in Group F was significantly increased compared with that of the control group. The collagen layers of the other groups that received soybean extract treatment were thicker than those of the control groups, but the differences were not significant. The number of estrogen receptor-positive cells in the skin of rats that received soy isoflavones obtained using the three different extraction methods was significantly decreased compared with that in the controls, but the differences among the groups that received the three different total soybean extracts were not statistically significant.
These results indicate that oral intake of the three different total soybean extracts might have similar effects on changes in the skin that are associated with aging.
One limitation of this study was that we did not compare the effects of daidzin, daidzein, genistin and genistein with those of the total soybean extracts. Therefore, further studies are needed to compare the efficacies of different total soybean extracts and soy-derived molecules and different administration routes to establish the parameters for using such products for skin health.
ACKNOWLEDGMENTSIn this study, tissue materials were used in phd thesis (12.SAG. BIL. 05), supported by Afyon Kocatepe University.
AUTHOR CONTRIBUTIONSUyar B planned and directed the study and wrote the manuscript. Sivrikoz ON performed and commented on the histopathological experiments. Ozdemir U performed the animal study. Dasbasi T performed and commented on the chemical experiments. Sacar H contributed to the writing of the manuscript and the search for references. We declare that all authors sufficiently participated in the study.
No potential conflict of interest was reported.