to investigate the relationship between mechanical ventilation and mortality and the practice of mechanical ventilation applied in children admitted to a high-complexity pediatric intensive care unit in the city of São Paulo, Brazil.
DESIGN:Prospective cohort study of all consecutive patients admitted to a Brazilian high-complexity PICU who were placed on mechanical ventilation for 24 hours or more, between October 1st, 2005 and March 31st, 2006.
RESULTS:Of the 241 patients admitted, 86 (35.7%) received mechanical ventilation for 24 hours or more. Of these, 49 met inclusion criteria and were thus eligible to participate in the study. Of the 49 patients studied, 45 had chronic functional status. The median age of participants was 32 months and the median length of mechanical ventilation use was 6.5 days. The major indication for mechanical ventilation was acute respiratory failure, usually associated with severe sepsis / septic shock. Pressure ventilation modes were the standard ones. An overall 10.37% incidence of Acute Respiratory Distress Syndrome was found, in addition to tidal volumes > 8 ml/kg, as well as normo- or hypocapnia. A total of 17 children died. Risk factors for mortality within 28 days of admission were initial inspiratory pressure, pH, PaO2/FiO2 ratio, oxygenation index and also oxygenation index at 48 hours of mechanical ventilation. Initial inspiratory pressure was also a predictor of mechanical ventilation for periods longer than 7 days.
CONCLUSION:Of the admitted children, 35.7% received mechanical ventilation for 24 h or more. Pressure ventilation modes were standard. Of the children studied, 91% had chronic functional status. There was a high incidence of Acute Respiratory Distress Syndrome, but a lung-protective strategy was not fully implemented. Inspiratory pressure at the beginning of mechanical ventilation was a predictor of mortality within 28 days and of a longer course of mechanical ventilation.
Diseases of the respiratory system are highly prevalent among Brazilian children, especially those aged less than 5 years. The World Health Organization Statistical Information System (WHOSIS) reported that Brazilian under-5 mortality rate, in 2000, was still 30/1000 live births. 13% of them have died from pneumonia alone.1
Specifically in the city of São Paulo, there is an increase of respiratory diseases. While in the 80’s 29% of children younger than age 5 presented respiratory diseases, with or without wheezing, in the 90’s the number increased to 49.6%.2
In many cases of respiratory diseases the use of ventilatory support is required. Besides that, ventilatory support may be needed in other situations, such as in sepsis and septic shock, neuromuscular diseases, during postoperative state and in cases of altered mental status with loss of consciousness. Thus, mechanical ventilation (MV) has become one of the major indications for admission to intensive care units.
Although MV benefits are unquestionable, its use can also cause harm. The adverse consequences of MV include airway lesions by intubation, pneumonia/sinusitis associated with MV, volutrauma, barotrauma, atelectrauma, and biotrauma. From the 70’s until the late 90’s, supraphysiologic tidal volumes (> 10ml/kg), and low or even zero positive end expiration pressures (PEEP) were used, with high mortality rates observed3. In the last years, technical devices in the intensive care units had improved and better ventilatory strategies have been introduced.
According to the lung-protective ventilation strategy, tidal volumes lower than 8ml/kg, PEEP high enough to permit FiO2 less than 0.6 and oxygen saturation (SatO2) above 88%, besides limiting plateau pressure, are indicated to achieve better outcomes, especially lower mortality rates, in patients with ARDS and ALI.4,5 Retrospective studies in infants and children suggest that the implementation of lung protective strategies could also be lowering the mortality rate. Albuali and coworkers showed that mortality decreased by 40% over the last 15 years, as lung-protective strategies have been used. In the study, higher tidal volume was independently associated with increased mortality and decreased ventilation-free days.6
Although data exist that 17 to 64% of all children admitted to PICUs have been mechanically ventilated, no epidemiologic study of this phenomena have been made in Brazilian children.7–11 Two multicenter studies, the International Group for Mechanical Ventilation in Children (IGMVC) and the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) showed that children on MV have a median age of 1 year, are ventilated for 6 or 7 days and frequently use more than one ventilatory mode. Prevalence of ARDS was low in both multicenter studies: 2% in the IGMVC study and 7.6% in the PALISI investigation. The PALISI trial also showed that 52.5% of children had a PaO2/FiO2 ratio of 200 or less for at least one day and that some of these may represent underrecognized cases of ARDS.7,8
It is unclear whether these results apply to our population in Brazil, since our population may be different and the current practice of mechanical ventilation is not known. Thus, in this study we investigated the practice of mechanical ventilation adopted in children admitted to a high-complexity pediatric intensive care unit (PICU) in the city of São Paulo, Brazil. To investigate the relationship between mechanical ventilation and mortality, we searched for risk factors that may have affected mortality within 28 days of admission to the PICU, and days on MV.
METHODSThe Instituto da Criança Pedro de Alcântara is the reference hospital for high-complexity pediatric diseases and is affiliated with São Paulo State University. It is a 135-bed pediatric hospital, with 13 beds in the PICU, at the time of our study.
The institutional review board approved the study before data collection began and waived informed consent, as the study was observational only. In order to collect data that were representative of current practice, the PICU medical team knew researchers had authorization to medical records, but only the PICU coordinator and the researchers were not blinded to what data were been collected.
All children admitted to the Instituto da Criança PICU from October 1st, 2005 to March 31st, 2006, who underwent MV for 24 hours or more, were included in the study. Exclusion criteria were the following: lack of arterial blood gas measurement, patients using ventilator devices without a tidal volume measurement, noninvasive ventilation, previous dependence upon MV, and oxygenation impairment by heart disease or pulmonary hypertension. Patients ventilated in another institution for more than 24 hours and transferred to our PICU were also excluded from the study. Two patients were on high frequency oscillatory ventilation, after a time of pressure ventilation. Only data from pressure ventilation were analyzed in these two, because of the inability to compare ventilatory variables.
Demographic data (gender, age, weight), date of admission, chronic functional status, date of initiating MV, access to airway, reason for MV and ventilator data were prospectively collected by two pediatric intensivists (DCBS, AROS), in forms based on that created by the IGMVC and adapted to our study.7 Exhaled tidal volume measured by the ventilator device was used, as not all endotracheal tubes were cuffed.
Table 1 lists all variables used in this study, as formerly used by the IGMVC Study. The variables were studied at two different times: T0 corresponded to the beginning of MV (first arterial blood gas measurement after MV start) and T1 to 48 hours post MV (arterial blood gas measurement closer to 48 hours of the institution of MV).
Ventilatory settings, arterial blood gas measurements and organ dysfunctions of children submitted for 24 hours of MV or more in a reference Brazilian PICU, from October 1st, 2005 to March 31st, 2006
| Variable | Median | P25 – P75 |
|---|---|---|
| Days of MV | 6.50 | 2.50 – 10.50 |
| Tidal volume (ml/kg) at T0 | 10 | 9–11 |
| Tidal volume (ml/kg) at T1 | 9.5 | 8.19 – 10.81 |
| PEEP at T0 | 8 | 6–10 |
| PEEP at T1 | 8 | 6–10 |
| RR at T0 | 20 | 17.5 – 22.5 |
| RR at T1 | 20 | 15.5 – 24.5 |
| Inspiratory pressure at T0 | 24 | 20.37 – 27.62 |
| Inspiratory pressure at T1 | 24 | 19.25 – 28.75 |
| pH at T0 | 7.36 | 7.27 – 7.45 |
| pH at T1 | 7.38 | 7.32 – 7.49 |
| PaO2/FiO2 at T0 | 245.1 | 159.11 – 331.08 |
| PaO2/FiO2 at T1 | 237.0 | 146.76 – 327.23 |
| OI at T0 | 6 | 3.51 – 8.49 |
| OI at T1 | 5.8 | 3.81 – 7.79 |
| PELOD | 11 | 6 – 16 |
| Organ dysfunctions at T0 | 2 | 1.5 – 2.5 |
| Organ dysfunction at T1 | 2 | 0.5 – 3.5 |
MV: mechanical ventilation; PEEP: positive end expiratory pressure; RR: respiratory rate; PaO2/FiO2: arterial partial pressure of oxygen / inspired fraction of oxygen ratio; OI: oxygenation index (mean airway pressure × FiO2 × 100 / PaO2); PELOD: Pediatric Logistic Organ Dysfunction.
The Pediatric Logistic Organ Dysfunction (PELOD) was used as a prognostic index.12 The outcomes analyzed included days of MV and mortality within 28 days after admission. To complete the outcomes analysis, all medical records were reviewed after patients were discharged from the hospital.
Arterial blood punctures were done without topical anesthesia. However, rapid sequence intubation was routinely performed with pre-oxygenation, administration of a sedative agent, an analgesic, and a paralyzing agent. Thus, when the arterial blood puncture was made, patients had already received these agents for 30 minutes to two hours prior the procedure. It was also part of standard care to keep ventilated patients under continuous sedation and analgesia, usually with midazolam and fentanyl. Blood punctures were performed on patients after they had received these agents.
The standard ventilator devices of the PICU were used, in which, besides expiratory tidal volume measurement, the following modes were available: pressure control ventilation (PCV), volume control (VCV), volume target pressure control (VTPC), synchronized intermittent mandatory ventilation (SIMV) with pressure support (PS) and spontaneous (Newport E500, Newport Medical Instruments, Inc., Newport Beach, CA, USA).
Statistical analysis was performed with StatView for Windows 5.01 software (SAS Institute, Cary, NC, USA). Categorical data are expressed as absolute counts and percentages. Continuous data are expressed as medians and interquartile range. Data were considered significant at p<0.05. The Mann- Whitney U test was used for non-paired continuous data, for two groups (survivors x nonsurvivors). Risk factors were identified by simple regression (univariate analysis) and their association power was measured by their odds ratios, considering the respective 95% confidence intervals.
RESULTSA total of 241 patients were admitted to the PICU between October 1st, 2005 and March 31st, 2006, with 86 undergoing mechanical ventilation for 24 hours or more. Of the 86, 37 met exclusion criteria, resulting in data from 49 eligible patients being analyzed (table 1). Participants had a median age of 32 months (P25; P75: 1.87; 62.12) and median weight of 13 kg (P25; P75: 7.77; 18.22). A large majority of the sample was male (61.23%). No patient data was lost.
Only 4 of 49 patients (8.16%) had no chronic status. The most prevalent chronic diseases were as follows: respiratory (n=13; 26.53%), neurological (n=9; 18.37%), hepatic (n=6; 12.24%) and oncological (n=7; 14.28%).
The major indication for MV in the 49 patients eligible for this study was acute respiratory failure (n= 29, 59.18%), followed by acute decompensation of chronic pulmonary disease (n= 11, 22.45%) and a lowered level of consciousness (n=8, 16.33%). Among patients with acute respiratory failure, 62% (n=17) had severe sepsis / septic shock.
The airway was achieved by orotracheal intubation, in most cases. There was no nasotracheal intubation. A total of 5 patients were previously tracheostomized, while another 3 were tracheostomized after 3 weeks of MV.
No child was ventilated by VCV. Of the total, 48 were ventilated by pressure modes and one was ventilated by volume-targeted pressure. A total of 23 patients (47%) received only one ventilatory mode (PCV or PS with SIMV) and 26 (53%) received more than one mode.
Weaning and extubation were not focused in this study, as they are included in another investigation currently underway.
Although no patients had an inspiratory pressure above 30 cmH2O at T0, at T1 9 patients had it. At T1, 23 patients had a tidal volume of 10 ml/kg or more; instead of that, no barotrauma was observed (Figure 1).
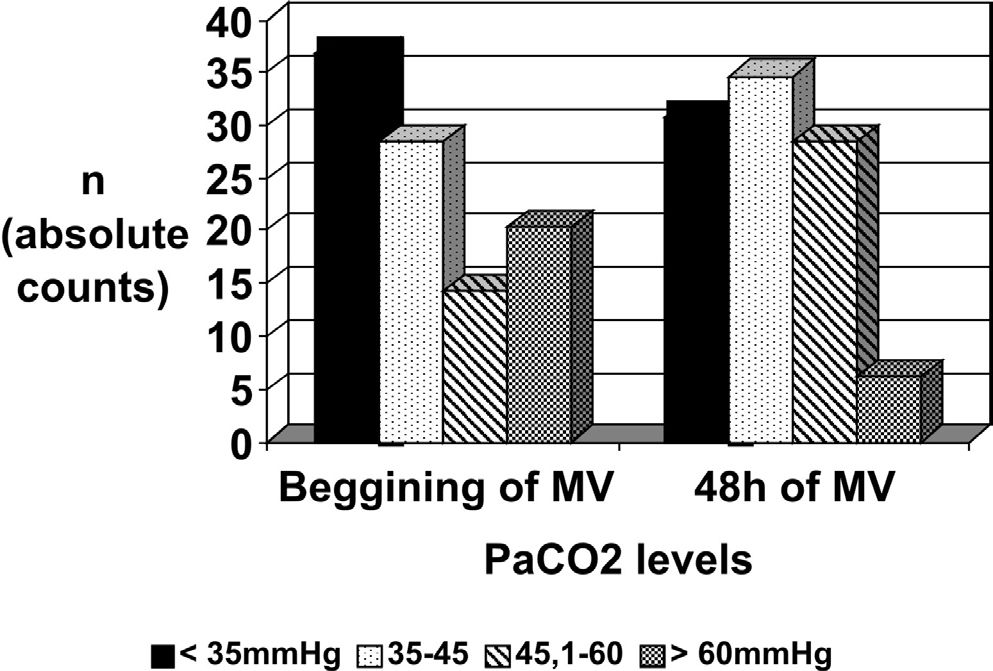
Most patients were normo or hypocapnic (Figure 2). A total of 41 patients had clinical, PaO2/FiO2 ratio and radiological data compatible with an ARDS diagnosis (n=25) or with acute lung injury non-ARDS (n= 16), in the first 48 hours of MV (13).
A median PELOD score of 11 was calculated, which corresponded to a 1.3% theoretical mortality. However, 28 days after admission to the PICU, 17 patients had died (34.69%).
As risk factors for mortality, we identified inspiratory pressure (p= 0.005), pH (p =0.049), PaO2/FiO2 (p=0.016) and oxygenation index at T0 (p= 0,0007), as well as oxygenation index at T1 (p= 0.011). PEEP and TV at T0 were not detected as risk factors. Table 2 shows the odd ratio of each risk factor for mortality and for staying more than 7 days on MV.
Risk factors for mortality and for staying more than 7 days on MV, with the respective odd ratio in each case, of children submitted for 24 hours of MV or more in a reference Brazilian PICU, admitted from October 1st, 2005 to March 31st, 2006
| Risk factor | OR for Mortality (95% IC) | OR for MV above 7 days (95% IC) |
|---|---|---|
| Initial inspiratory pressure > 25 cmH20 | 5.5 (1.53–19.71) | 5.96 (1.69– 21.03) |
| Initial tidal volume > 8 ml/kg | 1.9 (0.30–3.94) | 1.77 (0.50– 6.31) |
| Initial pH < 7.20 | 6 (1.26–28.55) | 1.88 (0.44– 8.07) |
| Initial PaO2/FiO2 < 200 | 2.14 (0.65–7.13) | 2.15 (0.65– 7.13) |
| Initial PaO2/FiO2 < 100 | 10.67 (1.08–105.29) | 6.35 (0.65–61.73) |
| Initial OI > 10 | 6.77 (1.46–31.30) | 4.16 (0.93–18.72) |
| Initial OI > 14 | 16.91 (1.83–156.62) | —— |
| OI at 48h of MV > 10 | 4.83 (0.97–23.98) | —— |
| OI at 48h of MV > 14 | 11.27 (1.13–112.07) | 4.06 (0.7– 23.47) |
Univariate analysis (simple regression) showed a correlation between days of MV and inspiratory pressure at T0 (p 0.015; R2 0.130), as also between days of MV and PaO2/FiO2 ratio at T0 (p 0.040; R2 0.111). But when the odds ratios were calculated, only initial inspiratory pressure was predictive of MV of more than 7 days, with an OR of 5.96 (IC 95% 1.69 – 21.03) (table 2).
DISCUSSIONThe “Instituto da Criança” is a high-complexity pediatric hospital, affiliated with São Paulo State University. Children who are admitted to the PICU are usually chronically ill and treated by pediatric subspecialties from the institute itself.14,15 Thus, one weakness of this study is it is not representative of the ventilatory practices of all Brazilian PICUs, but it may reflect the patterns of PICUs at other reference institutions in the country. Other weakness is that data extraction was performed around three years ago and practice could change in this time. Nevertheless, its major strength is to be the first Brazilian report of practice of mechanical ventilation in pediatric intensive care setting.
In this study, 35.7% of admitted patients underwent mechanical ventilation for 24 hours or more. This finding agrees with that described by others.7–11 Patients ventilated for less than 24 hours were usually of immediate postoperative status, those who had low level of consciousness that was rapidly reversed, and those who died within 24 hours after PICU admission.
The major indication for MV was acute respiratory failure (59.18%), generally associated with severe sepsis/septic shock (17/29, 58.62% of children with acute respiratory failure).
As pressure based ventilatory modes were mostly used, even though other modes were available, we can assume that our standard ventilatory pattern is pressure ventilation. The most used modes were PS + SIMV and PCV. Criteria for using PCV were severity of illness—the more severe the illness, the greater the necessity for resting the respiratory muscles.
As pressure ventilation was used, and no volume ventilation at all, we can assume more attention was paid to inspiratory pressure limits than to tidal volume control, even though both measures were available.
Concerning selection of ventilatory settings, it′s difficult to compare them among patients with different pathologies7. So, we compare settings in ALI patients only.
A total of 25 of our patients fulfilled the ARDS criteria, with an overall incidence of 10.37% of total admissions during the period. This finding differs from that observed in the studies by Randolph8 and Farias7, where the incidence of ARDS was 7.6% and 2%, respectively. This elevated incidence found in our study was not surprising, as 91.83% of our population presented a chronic functional status, which is clearly identified as a risk factor for ARDS.16 Besides that, our diagnosis of ARDS was done by active search, given the prospective profile of the study.
The PALISI trial showed that 52% of patients with PaO2/FiO2 of 200 or less, for at least 1 day, which could correspond to transitory hypoxemia, was an indication for developing ARDS during PICU stay or represented ARDS underrecognition.8 We expected to find hypercapnia, consistent with lung-protective ventilation.3–6, 16. However, most patients presented normo or hypocapnia, showing that we still underuse this life-saving ventilatory strategy.
In ARDS and ALI patients, risk factors for mortality and/or prolonged mechanical ventilation have been identified, which include the following: initial pH,17,18 PaO2/FiO2, 17,19tidal volumes higher than 8ml/kg,4,5,17 initial PEEP,17 initial inspiratory pressure and oxygenation index.17,20 In our population, we also identified inspiratory pressure, pH, PaO2/FiO2, oxygenation index at T0 but only oxygenation index at T1 of MV. No difference PEEP and tidal volume between survivors and non-survivors was observed. It is possible that such difference exists but the present study had insufficient statistical power to detect it.
Mortality 28 days after admission was at around 35%, well above the 1.3% expected by PELOD. Survival could be influenced by organ failure, immunocompromise and perhaps by the development of complications associated to the ventilatory strategies employed. It is also known that many models (PIM, PIM2, PRISM) underestimate mortality21–22. We used the PELOD score but could not say it underestimates mortality as well, as only a subpopulation of children admitted to the PICU was studied. Besides, this study did not aim to evaluate correlation and agreement between predicted and actual mortality by PELOD.
Specifically in “Instituto da Criança” PICU, the entire medical team is well familiar with the concept of ARDS/ALI and its treatment. Although adequate ventilator devices are available in order to implement an adequate ventilatory strategy, there is no written protocol to do so, allowing for personal variations in compliance with the strategy. It is also possible that high PaCO2 makes medical teams uncomfortable and that respiratory rates are elevated to compensate for the hypercapnia seen.
Many studies have tried to explain the reasons for the underuse of protective lung strategies in adult intensive care units.23–26 These includes: concerns over patient discomfort and tachypnea, hypercapnia and acidosis, worsening oxygenation, perception of contraindications to low tidal volumes, disease unrecognition 27, and assistance team being uncomfortable with low tidal volumes (especially for the risk of atelectasis).
Wolthuis and colleagues studied tidal volume use in three adult ICUs in the Netherlands to determine the effect of feedback and education concerning the use of lung protective MV. They have found that education did improve physician compliance in the use of lung protective ventilatory strategies, with a tidal volume decline within 6 months after intervention from 9.8 ml/kg ± 2.0 at baseline to 8.1 ±1.7 ml/kg.28 The feedback and education program focused on lung-protective MV, with an emphasis on adjusting tidal volumes to predicted body weight (PBW).
In our study we too observed that protective lung strategies for ALI/ARDS were not fully implemented, as ventilatory settings resulting in normocapnia / hypocapnia were still being used. It is possible that written protocols and feedback orientations, as well as an education program may improve compliance with lung-protective strategies. Thus, we suggested that such strategies be implemented as an attempt to make better use of MV and to reduce mortality rates.
CONCLUSIONSHere we showed that pressure ventilation (controlled and/or pressure support) was the ventilatory mode of choice used in this facility. Inspiratory pressures over 25 cmH2O were predictive of mortality (OR 5.5), as was MV duration of longer than 7 days (OR 5.96). Initial PaO2/FiO2 ratio, pH and oxygenation index, as oxygenation index at 48 h, were predictive of mortality. Protective lung strategies for ALI/ARDS were not fully implemented, as ventilatory settings resulting in normocapnia / hypocapnia were still being used.