During orthotopic liver transplantation for fulminant hepatic failure, some patients may develop sudden deterioration of cerebral perfusion and oxygenation, mainly due to increased intracranial pressure and hypotension, which are likely responsible for postoperative neurological morbidity and mortality. In the present study, we hypothesized that the favorable effects of hypertonic saline solution (NaCl 7.5%, 4 mL/kg) infusion on both systemic and cerebral hemodynamics, demonstrated in laboratory and clinical settings of intracranial hypertension and hemorrhagic shock resuscitation, may attenuate the decrease in cerebral perfusion pressure that often occurs during orthotopic liver transplantation for fulminant hepatic failure.
METHODS:10 patients with fulminant hepatic failure in grade IV encephalopathy undergoing orthotopic liver transplantation with intracranial pressure monitoring were included in this study. The effect on cerebral and systemic hemodynamics in 3 patients who received hypertonic saline solution during anhepatic phase (HSS group) was examined, comparing their data with historical controls obtained from surgical procedure recordings in 7 patients (Control group). The maximal intracranial pressure and the corresponding mean arterial pressure values were collected in 4 time periods: (T1) the last 10 min of the dissection phase, (T2) the first 10 minutes at the beginning of anhepatic phase, (T3) at the end of the anhepatic phase, and (T4) the first 5 minutes after graft reperfusion.
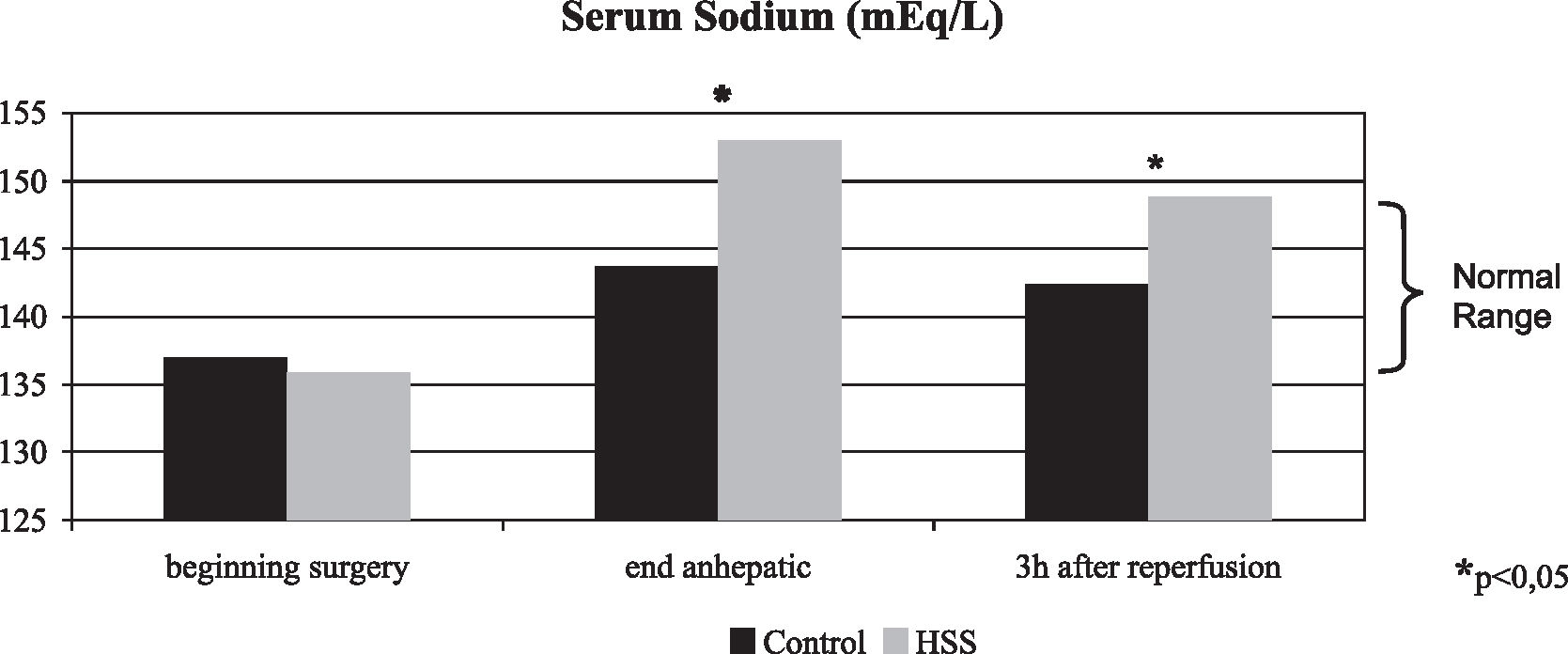
RESULTS:Immediately after hypertonic saline solution infusion, intracranial pressure decreased 50.4%. During the first 5 min of reperfusion, the intracranial pressure remained stable in the HSS group, and all these patients presented an intracranial pressure lower than 20 mm Hg, while in the Control group, the intracranial pressure increased 46.5% (P < 0.001). The HSS group was the most hemodynamically stable; the mean arterial pressure during the first 5 min of reperfusion increased 21.1% in the HSS group and decreased 11.1% in the Control group (P < 0.001). During the first 5 min of reperfusion, cerebral perfusion pressure increased 28.3% in the HSS group while in the Control group the cerebral perfusion pressure decreased 28.5% (P < 0.001). Serum sodium at the end of the anhepatic phase and 3 hours after reperfusion was significantly higher in the HSS group (153.00 ± 2.66 and 149.00 ± 1.73 mEq/L) than in the Control group (143.71 ± 3.30 and 142.43 ± 1.72 mEq/L), P = 0.003 and P < 0.001 respectively.
CONCLUSION:Hypertonic saline solution can be successfully used as an adjunct in the neuroprotective strategy during orthotopic liver transplantation for fulminant hepatic failure, reducing intracranial pressure while restoring arterial blood pressure, promoting sustained increase in the cerebral perfusion pressure.
Neste estudo testamos a hipótese de que os efeitos benéficos decorrentes da administração da solução salina hipertônica (NaCl 7,5%, 4 mL/kg) sobre a hemodinâmica sistêmica e cerebral na hipertensão intracraniana e no choque hemorrágico, possam atenuar a diminuição da pressão de perfusão cerebral que freqüentemente acompanha o transplante do fígado para hepatite fulminante.
MÉTODO:Foram estudados 10 pacientes com hepatite fulminante em encefalopatia grau IV e monitorização de pressão intracraniana submetidos ao transplante do fígado. A hemodinâmica sistêmica e cerebral de 3 pacientes que receberam solução salina hipertônica durante a fase anepática (Grupo SSH) foi analisada comparando com os dados obtidos de 7 pacientes transplantados anteriormente nas mesmas condições (Grupo Controle). Os valores de pressão intracraniana máxima e a correspondente pressão arterial média foram coletados em quatro tempos: (T1) nos últimos 10 min da fase de disseccão, (T2) nos primeiros 10 minutos da fase anepática, (T3) no final da fase anepática e (T4) nos primeiros 5 min da reperfusão
RESULTADO:Imediatamente após a infusão da solução salina hipertônica a pressão intracraniana diminuiu 50,4%. Nos primeiros 5 min da reperfusão a pressão intracraniana no Grupo SSH se manteve estável e todos os pacientes apresentavam pressão intracraniana menor que 20 mmHg enquanto no Grupo Controle a pressão intracraniana aumentou 46,5% (p<0,001). O Grupo SSH apresentou maior estabilidade hemodinâmica, nos primeiros 5 min da reperfusão hepática, a pressão arterial média no Grupo SSH aumentou 21,1% e no Grupo Controle diminuiu 11,1% (p<0,001). Nos primeiros 5 min da reperfusão a pressão de perfusão cerebral no Grupo SSH aumentou 28,3% e no Grupo Controle diminuiu 28,5% (p< 0,001). A natremia no final da fase anepáica e após 3 horas da reperfusão foi significativamente maior no Grupo SSH (153.00 ± 2.66 and 149.00 ± 1.73 mEq/L) que no Grupo Controle (143.71 ± 3.30 and 142.43 ± 1.72 mEq/L), p=0.003 e p< 0.001 respectivamente.
CONCLUSÃO:Estes resultados sugerem que a solução salina hipertônica pode ser utilizada com sucesso como medida neuroprotetora no transplante de fígado para hepatite fulminante, promovendo diminuição efetiva da pressão intracraniana e estabilidade cardiocirculatória, proporcionando aumento sustentado da PPC durante a cirurgia.
Fulminant hepatic failure (FHF) is a dramatic condition defined by the development of encephalopathy and coagulopathy complicating acute liver injury.1 Fulminant hepatic failure results in progressive multi-organ failure with a dramatic impact in the brain. Brain edema is a frequent finding, occurring in up to 80% of patients with grade IV encephalopathy, which ultimately leads to intracranial hypertension (IH). These conditions are major causes of death in FHF.2,3 Many articles have highlighted the close relationship between the process that results in brain edema and the pathogenesis of hepatic encephalopathy (HE).3–5 The management of patients with FHF is aimed mainly in preventing or reversing increased intracranial pressure (ICP) associated with support treatment for other failed organs. The definitive treatment for patients with FHF is liver replacement.
During orthotopic liver transplantation (OLT), patients may develop sudden deterioration of cerebral perfusion and oxygenation, mainly due to the combination of increased intracranial pressure and hypotension, despite aggressive monitoring and therapy.6–8 All current treatment modalities are far from perfect and are associated with serious adverse events:9–12 indiscriminate hyperventilation can lead to brain ischemia; mannitol can cause renal insufficiency and rebound ICP elevation; and barbiturates are associated with cardiovascular depression and prolonged coma.13 Failure to regain consciousness or occurrence of significant postoperative neurological sequelae despite satisfactory graft function has been reported,2,14,15 and may be caused by further brain damage from events occurring during surgery, indicating that peritransplantation neuroprotection must be improved.
Contemporary interest in hypertonic saline solution (HSS) was triggered in 1980, when Velasco et al16 demonstrated that a bolus injection of 7.5% NaCl (4 mL/kg) rapidly restored arterial pressure in severely hemorrhaged dogs. In animal experiments, HSS consistently has been shown to reduce ICP consequent to head trauma.17 In clinical trials, the beneficial effects of HSS during hemorrhagic shock in the presence of intracranial hypertension associated with systemic hypotension have been noted.18,19 Increasing evidence suggests important advantages in HSS resuscitation, namely, reduction in postresuscitation complications such as renal failure, coagulopathies, and acute respiratory distress syndrome, possibly by modulation of the systemic inflammatory response.20–22 The findings that HSS induces favorable effects on both systemic and cerebral hemodynamics in laboratory and clinical settings of IH suggest a potential use of this solution during OLT for FHF. This study presents the preliminary results of the use of HSS during OLT for FHF.
PATIENTS AND METHODSTen patients with FHF undergoing OLT with ICP monitoring were included in this study. Systemic and cerebral hemodynamics effects of HSS infusion in 3 patients (HSS group) were compared to the data obtained from historical controls in 7 patients (Control group) who underwent liver transplants between January 1999 and December 2004. Only patients who had stage IV encephalopathy and at least 1 episode of IH (defined by ICP >20 mm Hg) before surgery were included in this study. The local hospital ethics committee (CAPPesq HCFMUSP) approved the study protocol, and in the HSS group, written informed consent was obtained from next of kin.
The HSS group received HSS (NaCl 7.5%, 4 mL/kg) through a central line over a period of 10 minutes at the beginning of portal vein anastomosis. One of these patients (patient 2) received a half dose (2 mL/kg) at the beginning of anhepatic phase to control refractory IH occurred during native liver removal, and the remaining 2 mL/Kg was given at the scheduled time.
Monitoring of ICP was performed by intraparenchymal device (Camino Systems, San Diego, CA, USA) with continuous recording during the operation. The maximal ICP and the corresponding mean arterial pressure (MAP) values were collected during the following time periods: (T1) the last 10 min of the dissection phase, (T2) the first 10 minutes at the beginning of anhepatic phase, (T3) at the end of the anhepatic phase, and (T4) the first 5 minutes after graft reperfusion. Serum sodium was analyzed at 3 moments: at the beginning of surgery, at the end of anhepatic phase, and 3 hours after reperfusion.
The aim of management was to maintain a mean ICP below 20 mm Hg and cerebral perfusion pressure (CPP = MAP – ICP) above 50 mm Hg. Therapy to control ICP (mannitol, thiopental, hyperventilation, and head-up position at 30°) was instituted whenever the mean ICP became greater than 25 mm Hg.
Liver transplantation was either performed with preservation of the recipient’s inferior vena cava during hepatectomy (“piggy-back” technique) or with resection of the recipient’s retrohepatic inferior vena cava without using veno-venous bypass (conventional technique). All livers were hypothermically preserved with iced Belzer UW cold storage solution (Bristol-Myers Squibb, Princeton, NJ, USA). Before reperfusion, liver grafts were flushed with 750 mL of lactated Ringer’s solution, via the portal vein. The hepatic artery was reconstructed after reperfusion.
After data collection, the corresponding CPP for each time period (T1 – T4) was calculated, and parameter evolution was analyzed as the difference between the time periods. Data are presented as mean and standard deviation (Table 1). Statistical analysis was performed by t test; P < 0.05 were taken to be statistically significant.
Mean Systemic and Cerebral Hemodynamic Values (mm Hg)
| HSS group Mean ± SD | Control group Mean ± SD | P | |
|---|---|---|---|
| Intracranial pressure end dissection | 28.7 ± 6.1 | 20.6 ± 6.5 | not significant |
| Intracranial pressure early anhepatic | 36.3 ± 12.5 | 20.4 ± 5.9 | 0.021 |
| Intracranial pressure end anhepatic | 18.0 ± 4.6 | 15.9 ± 3.0 | not significant |
| Intracranial pressure graft reperfusion | 18.0 ± 1.0 | 23.3 ± 7.4 | not significant |
| Mean arterial pressure end dissection | 71.7 ± 4.7 | 77.7 ± 4.4 | not significant |
| Mean arterial pressure early anhepatic | 61.7 ± 5.8 | 63.0 ± 6.1 | not significant |
| Mean arterial pressure end anhepatic | 71.0 ± 3.6 | 69.1 ± 8.8 | not significant |
| Mean arterial pressure graft reperfusion | 86.0 ± 5.3 | 61.4 ± 5.4 | <0.001 |
| Cerebral perfusion pressure end dissection | 43.0 ± 7.8 | 57.1 ± 9.5 | not significant |
| Cerebral perfusion pressure early anhepatic | 25.3 ± 16.6 | 42.6 ± 11.3 | not significant |
| Cerebral perfusion pressure end anhepatic | 53.0 ± 8.2 | 53.3 ± 10.6 | not significant |
| Cerebral perfusion pressure graft reperfusion | 68.0 ± 6.2 | 38.1 ± 9.8 | 0.001 |
In the 3 patients of the HSS group, the ICP decreased immediately after HSS infusion (36.3 ± 12.5 mm Hg at the beginning of anhepatic phase vs. 18.0 ± 4.6 mm Hg at the end of anhepatic phase) (Figure 1). In one patient (patient 2), a half dose of HSS administrated at the beginning of anhepatic phase lowered a mannitol refractory surge in ICP from 45 to 19 mm Hg. In this group, the ICP remained consistently below 25 mm Hg from HSS administration through graft reperfusion (18.0 ± 4.6 mm Hg after HSS infusion vs. 18.0 ± 1.0 mm Hg in the first 5 min after reperfusion), and no patient developed new episodes of intracranial hypertension (Table 1, Figure 1). During the first 5 minutes after reperfusion, all these patients presented ICP lower than 20 mm Hg.
In the Control group at the beginning of anhepatic phase, 3 patients presented IH and a CPP lower than the critical level of 40 mm Hg, requiring thiopental and mannitol therapy. Intracranial pressure and CPP promptly normalized in all treated patients; no other episodes of intracranial hypertension occurred during the anhepatic phase. Cerebral perfusion pressure improved slightly during the anhepatic phase (42.6 ± 11.3 mm Hg at the beginning of the anhepatic phase vs. 53.3 ± 10.6 mm Hg at the end of the anhepatic phase), and at the end of this phase, all patients presented an ICP below 25 mm Hg (Table 1, Figures 1 and 2). During reperfusion, all patients in this group presented a rise in ICP (15.9 ± 3.0 mm Hg at the end of anhepatic phase vs. 23.3 ± 7.4 mm Hg within the first 5 min of reperfusion) (Table 1, Figure 1). Three of these patients presented IH during reperfusion.
The HSS group presented a greater hemodynamic stability than the Control group within 5 minutes after graft reperfusion (MAP, 86.0 ± 5.3 and 61.4 ± 5.4 mm Hg, respectively, P < 0.001) (Table 1, Figure 3). Increased hemodynamic stability in the HSS group was also documented by the absence of postreperfusion syndrome, as determined by MAP < 60 mm Hg within the first 5 minutes after reperfusion (0/3 in the HSS group vs 3/7 in the Control group).
Cerebral perfusion pressure decreased in both groups at the beginning of the anhepatic phase (41.2% in the HSS group vs 25.4% in the Control group, NS). Cerebral perfusion pressure during the anhepatic phase rose 109.5% in the HSS group vs 25.1% in the Control group, P = 0.059. The improvement in CPP during the anhepatic phase was secondary to a decrease in ICP (50.4% in the HSS group vs 22.1% in Control group) associated with an increase in MAP (15.1% in HSS group vs 9.7% in the Control group). Reperfusion was accompanied by a further increase of 28.3% in CPP in the HSS group, while in the Control group there was a decrease of 28.5%, (P < 0.001) (Figure 2). During graft reperfusion, the HSS group had no ICP variation along with a MAP increase of 21.1%, whereas in the Control group the ICP increased 46.5% and MAP decreased 11.1% (Table 2, Figures 1 and 3).
Mean Systemic and Cerebral Hemodynamic Evolution (mm Hg)
| HSS group Mean ± SD | Control group Mean ± SD | P | |
|---|---|---|---|
| Intracranial pressure T2-T1 | 7.7 ± 17.2 | -0.1 ± 7.5 | not significant |
| Intracranial pressure T3-T1 | -10.7 ± 10.5 | -4.7 ± 4.6 | not significant |
| Intracranial pressure T3-T2 | -18.3 ± 8.6 | -4.6 ± 4.9 | 0.011 |
| Intracranial pressure T4-T1 | -10.7 ± 6.5 | 2.7 ± 3.7 | 0.003 |
| Intracranial pressure T4-T3 | 0.0 ± 4.0 | 7.4 ± 5.3 | not significant |
| Mean arterial pressure T2-T1 | -10.0 ± 10.4 | -14.7 ± 6.3 | not significant |
| Mean arterial pressure T3-T1 | -0.7 ± 7.1 | -8.6 ± 9.0 | not significant |
| Mean arterial pressure T3-T2 | 9.3 ± 6.0 | 6.1 ± 8.3 | not significant |
| Mean arterial pressure T4-T1 | 14.3 ± 10.0 | -16.3 ± 6.8 | <0.001 |
| Mean arterial pressure T4-T3 | 15.0 ± 5.0 | -7.7 ± 8.8 | 0.004 |
| Cerebral perfusion pressure T2-T1 | -17.7 ± 23.9 | -14.6 ± 11.9 | not significant |
| Cerebral perfusion pressure T3-T1 | 10.0 ± 15.9 | -3.9 ± 6.9 | not significant |
| Cerebral perfusion pressure T3-T2 | 27.7 ± 11.7 | 10.7 ± 11.0 | not significant |
| Cerebral perfusion pressure T4-T1 | 25.0 ± 12.8 | -19.0 ± 6.5 | <0.001 |
| Cerebral perfusion pressure T4-T3 | 15.0 ± 7.8 | -15.1 ± 4.7 | <0.001 |
T2-T1: difference between beginning anhepatic and end dissection valuesT3-T1: difference between end anhepatic and end dissection valuesT3-T2: difference between end anhepatic and early anhepatic valuesT4-T1: difference between graft reperfusion and end dissection valuesT4-T3: difference between graft reperfusion and end anhepatic values
All patients in the HSS group had an intraoperative rise in serum sodium. Serum sodium at the end of anhepatic phase and 3 hours after reperfusion was significantly higher in the HSS group (153.00 ± 2.66 and 149.00 ± 1.73 mEq/L) than in the Control group (143.71 ± 3.30 and 142.43 ± 1.72 mEq/L), P = 0.003 and P < 0.001, respectively. At 3 hours after graft reperfusion, all patients presented serum sodium levels close to normality (normal range, 135 – 148 mEq/L) (Figure 4).
In the HSS group, 1 patient developed primary graft nonfunction and died on the second postoperative day. The other 2 patients regained consciousness within first week after OLT without any neurological damage.
In the Control group, there was 3 early deaths. The 2 patients who experienced the lowest CPP during surgery died; one died 2 hours after reperfusion and the other 8 hours after surgery. The third patient never regained conscience despite satisfactory graft function and died from multiple organ failure. The remaining 4 patients regained conscience after OLT; 1 died from sepsis, and the other 3 made good recoveries.
DISCUSSIONThis study evaluated the effects of HSS infusion on cerebral hemodynamics in patients with FHF during OLT. In this study, HSS infusion led to an immediate decrease in ICP and to a marked rise in CPP. In all patients, the ICP remained consistently below 25 mm Hg from HSS administration through graft reperfusion, and no patient developed new episodes of IH. In our study, HSS proved to be effective in preventing and treating surges in ICP that often occur in patients with cerebral edema undergoing OLT. The progressive rise in CPP between HSS administration and graft reperfusion was supported by the reduction and maintenance of ICP within security levels combined with more favorable hemodynamic profiles during reperfusion.
Several centers that perform liver transplantation for FHF have observed that patients for whom ICP had been controlled prior transplantation experience recurrence of increased ICP during surgery despite aggressive monitoring and therapy. Intraoperative care requires judicious use of fluids and pressors to maintain hemodynamic profiles and avoid increases of ICP during the entire procedure. A decrease in CPP is observed during liver transplantation for FHF when a decrease in blood pressure and cardiac output may be associated with an increase in ICP.6–8 In FHF, a systemic inflammatory response syndrome occurs in most of the patients,23 thus complicating hemodynamic and ICP control during surgery. Failure to regain consciousness despite satisfactory graft function may be caused by further damage to the brain from these events that frequently occur during surgery.
Evidence has been accumulating from case series and small randomized trials, showing that HSS may be an effective treatment for brain edema and elevated ICP after head trauma.24,25 The beneficial effects of HSS during hemorrhagic shock in the presence of IH associated with systemic hypotension have also been noted.18,19 Interestingly, it was particularly useful to lower ICP in one of our patients who presented IH that was refractory to treatment with mannitol, confirming the already described effectiveness of HSS in lower ICP when previous therapy to control IH has failed,24,26–28 pointing to HSS as an alternative therapy when we did not consider reasonable to repeat a treatment that was not successful.
The primary mechanism of action of HSS therapy is rapid osmotic mobilization of cellular water into the blood volume. Increased cardiac effectiveness occurs because of a combination of increased preload (venous return) and reduced afterload (vasodilatation).29,30 Increased contractility also has been reported in several studies.31,32 Restoration of normal intravascular physiology by HSS is achieved through a potent osmotic gradient across the cell membrane that causes a net shift of fluid from the cell (mainly endothelial and red blood cells) through the interstitium into the intravascular space.22,30 This solution also decreases the swelling of most cell types including neural cells.33 The reflection coefficient for sodium chloride is 1.0 (that of mannitol is 0.9), and under normal conditions, sodium has to be transported actively into the cerebrospinal fluid.13,34 The effect of HSS on the peripheral vasculature and microcirculation is generally to induce changes that increase flow. Capillary perfusion may be further augmented by the ability of HSS to reverse specific cellular effects of ischemia-reperfusion.29 The systemic inflammatory response that occurs in ischemia-reperfusion states promotes cytokine release and increased reactive oxygen metabolites, which ultimately cause microvascular damage, all of which can be attenuated by HSS administration.22 Hyperosmolarity down-regulates the initial inflammatory activation of neutrophils and up-regulates immunologic protection provided by lymphocytes.29 Hepatocellular injury related to the process of ischemia-reperfusion may contribute to patient morbity following liver resection for tumors, trauma, and transplantation.35 HSS decreases liver tissue damage after hemorrhagic shock combined with ischemia and reperfusion of the liver.36 The effect of HSS on systemic inflammation may help to reduce the severity of intracranial hypertension.22,29
The reperfusion time during OLT is a critical period characterized by hemodynamic instability and may be associated with a high frequency of intraoperative cardiovascular complications.37 These complications may range in severity from a transient hypotension to an acute hemodynamic deterioration known as postreperfusion syndrome.38 In this study, infusion of HSS resulted in avoidance of postreperfusion syndrome in all patients. The arterial blood pressure attained levels even higher than those determined before the anhepatic phase without the need of supplementary vasoactive drugs. The net effect observed was the rise of 28.3% in CPP seen in the HSS group versus the 28.5% decrease in CPP observed in Control group.
In the present study, a bolus infusion of HSS consistently led to a decrease in the ICP of all patients while maintaining blood pressure and cardiac output. The restoration of arterial blood pressure along with reduced ICP are essential conditions for improving intraoperative CPP. The favorable nature of these effects was more remarkable during reperfusion, which has been demonstrated to be a period frequently associated with the risk of brain injury secondary to elevated ICP and low CPP.6–8 In our study, the beneficial effects of HSS infusion on CPP were sustained during the remaining surgical time, precluding the need for additional specific therapeutic measures to maintain CPP above critical levels.
Infusion of HSS may involve risks because it induces a rapid change in serum sodium concentration. Although central pontine myelinolysis is a hazardous complication, it is more likely to occur after rapid correction of chronic hyponatremia.39,40 In FHF, there is no chronic hyponatremia; therefore, there seems to be no contraindication for its use. Indeed, hypertonic solutions benefited patients with FHF by decreasing ICP.5 There has never been a single case, among more than 1,700 patients treated with HSS, of seizures, intracranial bleeding, central pontine myelinolysis, or neurological deterioration induced by the HSS.19,28 No patient in the present series presented neurological complications.
The effects on intracranial, arterial blood, and cerebral perfusion pressures are most remarkable shortly after the administration of HSS, but they can also be observed over several hours and therefore may be useful during OLT until liver function is completely restored. In patient 2, the half-dose administration at the beginning of the anhepatic phase was also followed by hemodynamic improvement, which was sustained even after total vena cava clamping, suggesting that the potential benefits of HSS administration prior to the anhepatic phase may result in avoidance of veno-venous bypass and high-dose hemodynamic pharmacologic support.
Despite the time factor for the Control group (7 patients studied in a 6-year period), the technological and clinical improvements during this period did not change the history of this disease, and controlling IH remains a major challenge in FHF that is even now based on classical medical management and with OLT still being the best option for these patients.
These results show that HSS can be a useful tool in patients with FHF during liver transplantation, as an adjunct neuroprotective measure that actually improves CPP, especially when classical measures for neuroprotection against brain ischemia have failed.
CONCLUSIONHypertonic saline solution can be successfully used in patients with fulminant hepatic failure during orthotopic liver transplantation, reducing intracranial pressure while restoring arterial blood pressure and cerebral perfusion pressure. Despite the limited number of patients and the absence of concurrent controls, these data highlight the need for controlled clinical trials evaluating its use as adjunct therapy for intracranial hypertension in fulminant hepatic failure and as first line therapy for neuroprotection during reperfusion in orthotopic liver transplantation for fulminant hepatic failure.