Refeeding syndrome occurs in patients with severe malnutrition when refeeding begins after a long period of starvation. This syndrome increases the risk of clinical complications and mortality. Hypophosphatemia is considered the primary characteristic of the syndrome. The aim of our study was to investigate the presence of other electrolyte alterations in patients with cancer during the early stage of refeeding.
METHODS:In this observational study, we enrolled 34 patients with cancer of the upper aerodigestive tract receiving upfront radiotherapy who were also enrolled in a nutrition program. A caloric intake assessment, anthropometric measurements and biochemical laboratory tests were performed.
RESULTS:Significant weight loss (∼20%) was found in these patients. In the patients receiving artificial nutrition, we found lower levels of potassium and total protein compared with those who were fed orally (p = 0.03 for potassium and 0.02 for protein, respectively). Patients on enteral tube feeding had a higher caloric intake compared with those who were fed orally (25±5 kcal/kg/day vs. 10±2 kcal/kg/day).
CONCLUSION:Hypokalemia, like hypophosphatemia, could be a complication associated with refeeding in patients with cancer. Hypokalemia was present in the early stages of high-calorie refeeding.
Refeeding syndrome (RS) is a common condition occurring in patients with severe malnutrition (1,2). RS is associated with an increased risk of clinical complications and mortality (1,2). The incidence of this condition remains unknown, given the heterogeneity of the studies on this issue and the fact that RS is frequently unrecognized. RS is characterized by electrolyte disorders, such as hypophosphatemia, acute vitamin B1 deficiency, volume overload, cardiac insufficiency and hyperglycemia.
Furthermore, it has been suggested that potassium and magnesium may also be altered in RS (3,4). These electrolytes become depleted during starvation. Successively, during refeeding, when these electrolytes enter cells, their serum levels further decline (5,6). Electrolyte disorders develop during the early phase of refeeding. These conditions could influence the clinical outcomes of patients because they increase the risk of complications (7). The aim of this study was to investigate the eventual presence of hypokalemia during the early phase of refeeding in patients with cancer. In particular, we investigated a population of patients affected by tumors of the upper aerodigestive tract (UADT), which are the most distressing cancers associated with a long survival period (8).
METHODSBetween 2009 and 2012, approximately 200 patients with different types of cancers underwent nutritional status examinations in the Clinical Nutrition Unit at the University Magna Grecia of Catanzaro. For this investigation, only patients having at least one UATD were enrolled. We included only subjects receiving upfront radiotherapy (RTx) and who had recently started a nutritional therapy (at least 1 week before enrollment) orally or as artificial nutrition. A total of 34 individuals were enrolled. The study protocol did not require institutional review board approval because the study was observational. The data were anonymous; all of the patients provided written consent to participate in the study, which was performed in accordance with the principles of the Declaration of Helsinki.
Nutritional intake and anthropometric measurementsAll of the tests were performed after a 12-h overnight fast. The participants' caloric intake was evaluated with an interview performed by a dietician during the early days of refeeding (within 1 week) and was calculated using MetaDieta nutritional software, version 3.0.1 (Metedasrl, S. Benedetto del Tronto, Italy). Body weight was measured before breakfast with the subjects lightly dressed, subtracting the weight of their clothes. Body weight was measured with a calibrated scale, and height was measured with a wall-mounted stadiometer. BMI was calculated with the following equation: weight (kg)/height2 (m2).
Bioelectrical impedance analysis (BIA) (BIA-101; Akernsrl, Florence, Italy) was performed to estimate total body water (TBW), fat mass (FM) and total fat-free mass (FFM) (9).
Handgrip strength was measured using a hydraulic hand dynamometer (Hersteller/manufactures; SAEHAN Corporation, Masan, Republic of Korea; Distributor Rehaforum Medical GmbH, Elmshorn, Germany) with the subjects seated and their elbows flexed at 90°. The handgrip strength value was considered to be the maximum amount of kilograms of force obtained during the test (10).
Skin fold thickness was measured at the triceps with the GIMA Skinfold Caliper (Gessate, Milan, Italy) (11). The site was measured three times, and the mean was calculated.
Venous blood was collected into vacutainer tubes (Becton & Dickinson) and was centrifuged within 4 h. For our purposes, only abnormalities in serum albumin, total protein, potassium and sodium were investigated, all of which were assessed by standard laboratory techniques (with protein electrophoresis and emission flame photometry for electrolytes) (12).
Statistical analysisData are reported as the mean ± S.D. We classified the population into three groups according to the route of nutrient intake/administration, i.e., oral nutrition (PO), enteral tube feeding (ETF) or total parenteral nutrition (TPN). Furthermore, we categorized the participants according to cancer type. The t-test and ANOVA were used to compare the means between groups. Significant differences were assumed to be present at p<0.05. All of the comparisons were performed using SPSS software, version 20.0, for Windows (Chicago, IL, USA).
RESULTSThe mean age of the population was 65±12years. A total of 82% of the population was male (n = 28). As expected, there were significant differences between genders with respect to handgrip strength (higher in men) and the number of examinations performed (higher in women) (p<0.001 and p = 0.038, respectively). Patients on PO had a mean caloric intake of 10±2 kcal/kg/day, while patients on ETF or TPN had a mean caloric intake of 25±5 kcal/kg/day.
The patient characteristics are shown in Table 1. In these patients, weight loss relative to the preceding 3-6 months was significant (19±10%).
Characteristics of the study population (total number of patients with UADT = 34).
| Variables | Mean±SD |
|---|---|
| Age (years) | 65.8±12 |
| BMI | 21.5±5 |
| Weight loss (%) | 19.2±10 |
| Triceps fold (cm) | 1±0.6 |
| Hand grip strength (kg) | 24±10 |
| Serum albumin (g/dl) | 3.6±0.5 |
| Sodium (mEq/l) | 136.3±3 |
| Potassium (mEq/l) | 3.9±0,5 |
| Serum total protein (g/dl) | 6.4±0.8 |
| TBW (%) | 61.3±7 |
| FFM (%) | 80.2±11 |
| FM (%) | 19.7±11 |
| Number of visits | 0.83±1 |
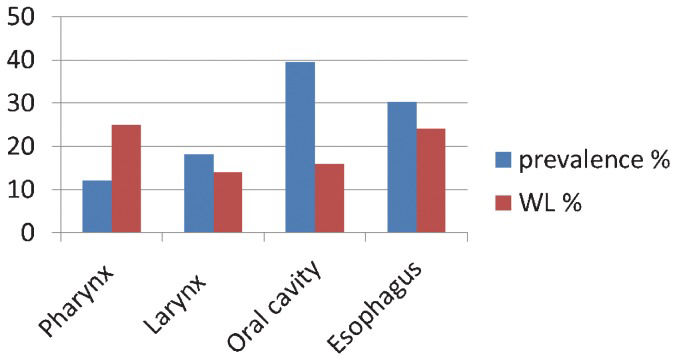
Figure 1 depicts the types and prevalence of UADT in the patient population. The figure shows greater weight loss in patients with pharyngeal and esophageal tumors compared with the weight loss in patients with other cancer types.
Table 2 shows the significant differences in serum potassium and total protein levels between patients receiving different routes of nutrient administration, with the lowest values found in ETF patients.
Characteristics of the population according to the route of nutrient intake.
| PO (48%) | ETF (34%) | TPN (18%) | p-value | |
|---|---|---|---|---|
| Age (years) | 67.1±12 | 65.3±10 | 65.8±14 | ns |
| BMI | 23.1±6 | 21±4 | 19.4±3 | ns |
| Weight loss (%) | 18.9±10 | 18.7±13 | 16.2±6 | ns |
| Albumin (g/dl) | 3.8±0.4 | 3.4±0.4 | 3.7±0.8 | ns |
| Sodium (mEq/l) | 136.8±2.9 | 136.5±4.2 | 135±2.4 | ns |
| Potassium (mEq/l) | 4.2±0.5 | 3.5±0.5 | 4.1±0.2 | 0.03 |
| Total protein (g/dl) | 7.1±0.5 | 4.6±0.1 | 6.2±0.3 | 0.02 |
| TBW (%) | 60.3±10 | 62.8±6 | 63.4±5 | ns |
| FFM (%) | 78.4±13 | 79.8±9 | 87±7 | ns |
| FM (%) | 21.6±13 | 20.1±9 | 13±7 | ns |
| Number of visits | 0.46±0.8 | 0.8±1 | 1±1 | ns |
In this study, we found lower potassium and total protein levels during the early phase of refeeding with artificial nutrition in patients with UADT compared with subjects who were fed orally (Table 3). In this population, we also found significant and worrisome weight loss (∼20%).
The relationship between cancer and malnutrition is well established. The percentage of patients with malnutrition is particularly high for gastrointestinal and head and neck cancers (13), as confirmed by our study. It is well accepted that enteral nutrition represents the most favorable nutritional approach because it can reduce hospital stays and medical complications (14). However, RS can occur as a result of the reintroduction of nutrients in patients with severe malnutrition or in starved patients on either ETF or TPN. Consequently, our study could play an important role in the recognition, education and prevention of RS. In fact, it is well known that glucose levels decline with starvation or under conditions of carbohydrate restriction (15). Consequently, non-carbohydrate sources (muscle proteins) are metabolized into glucose. In addition, in the hepatocytes, fatty acid oxidation can generate ketone bodies via the Krebs cycle. Under this condition, there is significant depletion of potassium, phosphate and magnesium, as well as losses of body fat and protein mass. However, a series of homeostatic mechanisms can maintain the concentrations of these ions at normal levels (16). During refeeding in great quantities, when a rapid increase in serum insulin occurs (15), the movement of extracellular potassium into the intracellular compartment can result in a dangerous decrease in potassium levels (15). Symptoms occur when the changes in serum electrolytes affect the cell membrane potential. Hypokalemia could be considered an early sign of RS, and it must be promptly corrected. To reduce the risk of developing RS, both enteral and parenteral feeding should be started at a reduced calorie rate (17). In fact, in our population, different amounts of caloric intake were found between the groups during the early phase of refeeding (Table 2). This finding confirms the effects of total energy administration on the development of hypokalemia and the risk of RS (17). It is likely that the higher caloric administration (mean caloric intake of 25±5 kcal/kg/day) in the ETF and TPF groups, compared with the PO group, was the cause of the lower levels of potassium.
The results of this study are important because RS is frequently unrecognized. Dietitians, nurses and physicians play important roles in reducing the risk of RS and improving the quality of life of these particular patients.
This study had some limitations that must be addressed. First, the results of this study should be interpreted with caution, as the study was not designed to assess the mechanisms of RS.
Furthermore, because of the serious clinical conditions of and limited collaboration obtained from these patients, we did not perform an accurate nutritional intake investigation to evaluate micronutrient intake. Finally, another limitation is the small sample size, although other studies on this topic used similar sample sizes [18,19].
However, this group of patients could be considered sufficiently homogeneous because all of the patients were affected by UADT and did not have significant clinical or biochemical differences (data not shown).
In conclusion, RS remains a current, unrecognized problem, and it includes electrolyte disorders, such as hypokalemia, during the early phase of refeeding. Because patients with UADT can have longer survival periods than other patients with cancer, dietitians, nurses and physicians could improve the quality of life of these particular patients by considering the complex biochemical manifestations of RS.
Grasso S, Ferro Y and Migliaccio V were responsible for the integrity of the data and the data collection. Mazza E and Rotundo S were responsible for the clinical and nutritional evaluations. Pujia A revised the manuscript and approved the final version. Montalcini T was responsible for the study design, data analysis and manuscript writing and approved the final version.
No potential conflict of interest was reported.