The present study aimed to evaluate the dynamics of CD28 and CD57 expression in CD8+ T lymphocytes during cytomegalovirus viremia in bone marrow transplant recipients.
METHODSIn a prospective study, blood samples were obtained once weekly once from 33 healthy volunteers and weekly from 33 patients. To evaluate the expression of CD57 and CD28 on CD8+ T lymphocytes, flow cytometry analysis was performed on blood samples for four months after bone marrow transplant, together with cytomegalovirus antigenemia assays.
RESULTSCompared to cytomegalovirus-seronegative healthy subjects, seropositive healthy subjects demonstrated a higher percentage of CD57+ and a lower percentage of CD28+ cells (p<0.05). A linear regression model demonstrated a continuous decrease in CD28+ expression and a continuous increase in CD57+ expression after bone marrow transplant. The occurrence of cytomegalovirus antigenemia was associated with a steep drop in the percentage of CD28+ cells (5.94%, p<0.01) and an increase in CD57+ lymphocytes (5.60%, p<0.01). This cytomegalovirus-dependent effect was for the most part concentrated in the allogeneic bone marrow transplant patients. The development of acute graft versus host disease, which occurred at an earlier time than antigenemia (day 26 vs. day 56 post- bone marrow transplant), also had an impact on the CD57+ subset, triggering an increase of 4.9% in CD57+ lymphocytes (p<0.05).
CONCLUSIONWe found continuous relative changes in the CD28+ and CD57+ subsets during the first 120 days post- bone marrow transplant, as part of immune system reconstitution and maturation. A clear correlation was observed between the expansion of the CD57+CD28−CD8+ T lymphocyte subpopulation and the occurrence of graft versus host disease and cytomegalovirus viremia.
Since the first bone marrow transplantations (BMTs) in the 1970s, many patients have benefited from their increasingly broad application.1 In recent years, more profound immunosuppression has been used to overcome problems associated with allogeneic BMTs and graft versus host disease (GVHD), and this has led to an increased frequency of life-threatening CMV infection in BMT recipients.2,3 Indeed, the incidence of CMV infection can reach 79% in CMV-seropositive recipients4 and 44% in CMV seronegative recipients with engraftment from CMV-seropositive donors.2–8
CD28, a co-stimulatory molecule present on the surface of T lymphocytes, is present in early stages of lymphocyte development and decreases after antigen stimulation.9,10 Its expression is, for the most part, mutually exclusive with that of CD57, which is expressed in 1 to 16.3% of circulating CD8+ T lymphocytes.11,12 Changes in CD28 and CD57 expression in CD8+ T lymphocytes have been reported to occur in association with a number of different conditions, including human immunodeficiency virus infection,11,13,14 rheumatoid arthritis,15 multiple myeloma,16,17 aging,18 BMT19 and allogeneic transplants, specifically.11 However, the most commonly reported circumstance resulting in CD28 downregulation and CD57 upregulation is CMV infection, either in isolation20,21 or in the setting of other conditions, such as HIV infection,22 aging,23 rheumatoid arthritis24 and stem cell transplantation.25 Different studies have suggested that increased CD57 expression and lack of CD28 expression in CD8+ T cells result from chronic antigen stimulation. These cells have been described as memory effector T-cells but lack a clear cytolytic function.26
The high incidence of CMV viremia and disease in BMT recipients makes these patients an ideal group in which to understand the relationship between CMV viremia and CD28 and CD57 expression in CD8+ T lymphocytes. Therefore, a prospective evaluation of these patients during the first 120 days after BMT was conducted, and the results suggested a correlation between CMV viremia and the expansion of the CD57+CD28−CD8+ T lymphocyte subset.
MATERIALS AND METHODSVolunteers and patientsThis study was approved by the Ethics Committee of the University of São Paulo Medical School, and written informed consent was obtained from all participants, according to the guidelines of the Brazilian Ministry of Health. Thirty-three healthy volunteers, including some bone marrow donors, constituted the preliminary control lymphocyte population. Nine were seronegative for CMV antibodies, while 24 were seropositive. No statistically significant differences in age or gender were observed between the seronegative and seropositive groups. One blood draw was performed to obtain an EDTA-sample for flow cytometric analysis.
Bone marrow transplant recipients at the BMT program of the Hematology and Hemotherapy Discipline at the University of São Paulo Medical School were prospectively enrolled in this trial. Thirty-three patients were eligible for the study. Twenty-two patients underwent allogeneic BMTs, and eleven patients received autologous BMTs. In the allogeneic BMT group, all patients received BMTs from a Human Leukocyte Antigen (HLA)-identical donor, and no patient received a T-cell depleted BMT. Of the 33 BMT recipients, only five (15.5%) were seronegative for CMV. All of these were allogeneic BMT recipients. Of the 22 allogeneic BMT recipients, only one (4.5%) involved a recipient and donor who were both CMV negative. Of the 11 autologous BMT recipients, none was seronegative for CMV (Table 1).
Patient demographics, BMT type, underlying disease, and immunosuppressive regimens used before BMT
| Gender | n (%) |
|---|---|
| Female | 20 (60.6%) |
| Male | 13 (39.4%) |
| Age | Years (range) |
| Mean | 29 (20–34) |
| BMT type | n (%) |
| Allogeneic | 22 (66.6%) |
| Autologous | 11 (33.3%) |
| Underlying disease | n (%) |
| Chronic myelogenous leukemia | 12 (36.4%) |
| Severe aplastic anemia | 5 (15.2%) |
| Acute myelogenous leukemia | 7 (21.2%) |
| Hodgkin’s lymphoma | 6 (18.2%) |
| Other* | 3 (9.1%) |
| Immunosuppressive conditioning regimen | n (%) |
| Busulfan and melphalan | 20 (60.6%) |
| Busulfan and cyclophosphamide | 3 (9.1%) |
| Total body irradiation and melphalan | 3 (9.1%) |
| Total body irradiation and cyclophosphamide | 2 (6.1 %) |
| Other | 5 (15.2%) |
| CMV serologic status pre-BMT | |
| Positive | 28 (84.8%) |
| Negative | 5 (15.2%) |
| Donors’ CMV serologic status | |
| Positive | 21 (95.5%) |
| Negative | 1 (4.5%) |
BMT: bone marrow transplant;
Blood samples were taken from BMT recipients once a week, beginning at the start of the conditioning period and continuing until 120 days after the BMT. Samples were processed for lymphocyte immunophenotyping and CMV antigenemia. After BMT, all of the patients underwent weekly clinical examination. Imaging exams were performed at the discretion of the attending physician.
DefinitionsActive CMV infection was defined as the presence of at least one episode of positive antigenemia. Positive antigenemia was defined by the detection of one or more peroxidase-positive (dark-brown nuclear staining) cells in 300,000 polymorphonuclear leukocytes. The presence of specific CMV antibodies was assessed by enzyme- immune-asay (EIA), before transplantation and every two weeks thereafter, as previously described.4 CMV disease was defined according to Ljungman et al.29 Diagnosis and clinical grading of acute GVHD were performed according to Thomas et al.30 For statistical analysis, GVHD was considered present when it was diagnosed as grade II or greater.
CMV surveillance and antiviral policiesThe pp65 detection (antigenemia) assay was performed according to Van der Bij et al.,27 with minor modifications.28 Whenever positive antigenemia was detected (≥ one positive cell/300,000 cells), the test was repeated every other day until the antigenemia resolved. When antigenemia of ≥ two positive cells was detected, patients were started on preemptive intravenous ganciclovir (GCV) therapy at a dose of 5 mg/kg, twice daily for 14 days. If antigenemia remained positive after seven days, GCV was continued through the complete 21 days of therapy, or until the antigenemia resolved. In patients with antigenemia of fewer than two positive cells, GCV was not started, and the patient’s antigenemia levels were closely monitored. No secondary prophylaxis with ganciclovir was routinely used after preemptive therapy, except in patients with two or more episodes of positive antigenemia. When CMV disease was diagnosed, patients were treated with GCV at the same dose used for preemptive therapy. No intravenous immunoglobulin was used to treat CMV disease.
All of the patients received HSV prophylaxis with acyclovir (400 mg, twice daily) until day 50 post-BMT. No prophylaxis for CMV disease was routinely used in this population.
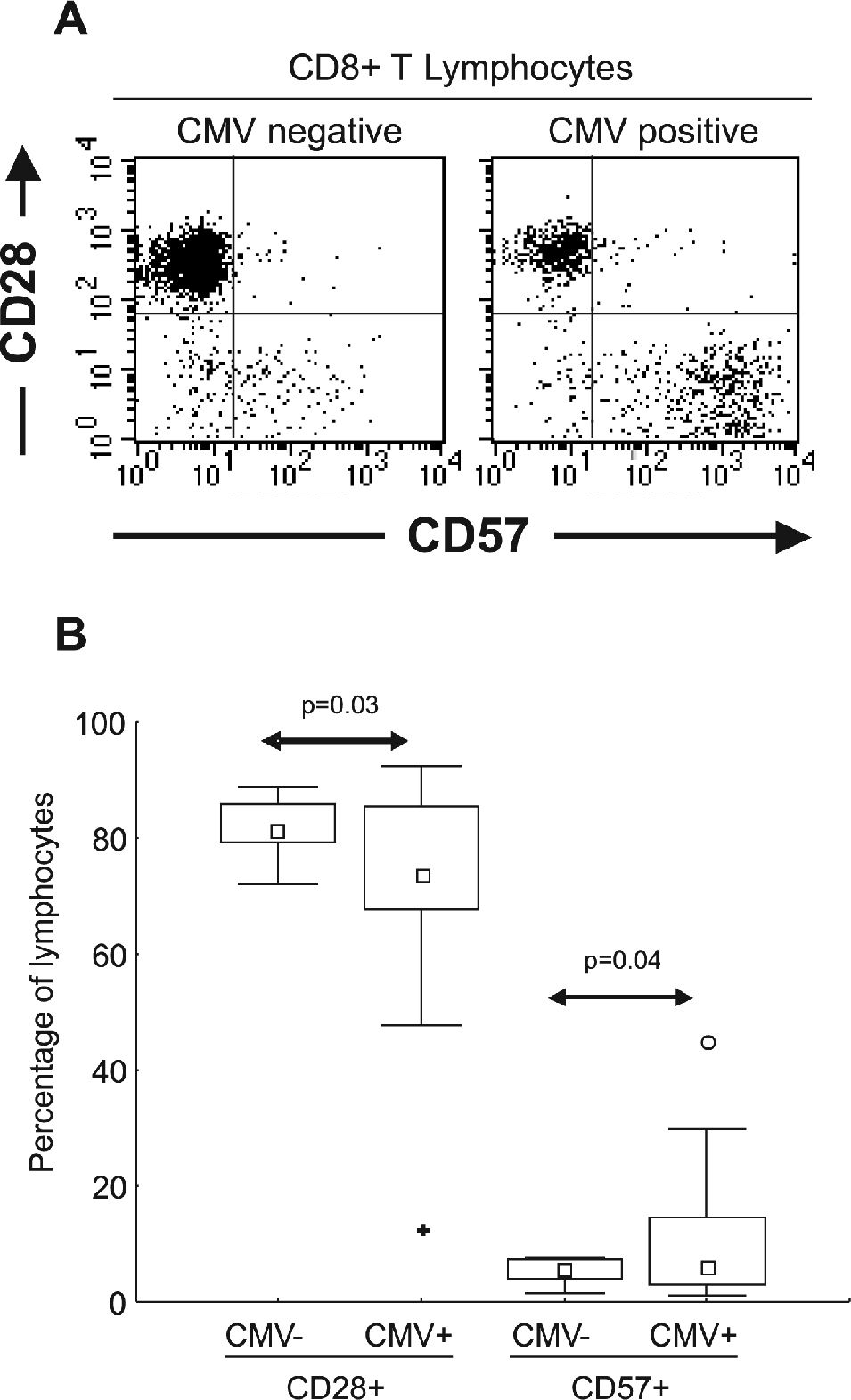
Monoclonal antibodies and flow cytometryCD57 fluorescein isothiocyanate (FITC, clone NK-1), IgM FITC (isotype control, clone G155-228), CD28 phycoerythrin (PE, clone CD28.2), IgG PE (isotype control, clone MOPC-21), CD8 Cy-Chrome (CyC, clone RPA-T8), and CD3 allophycocyanin (APC, clone UCHT1) monoclonal antibodies were obtained from PharMingen (San Diego, CA, USA). One hundred microliters of EDTA-treated blood were incubated at room temperature with a combination of monoclonal antibodies for 15 minutes in the dark and then treated with hemolysis buffer for ten minutes. Cells were washed and resuspended in phosphate-buffered saline (PBS) supplemented with 0.1% sodium azide for cytometric analysis. Cell samples were analyzed on a FACSCalibur flow cytometer (BDIS) equipped with argon and diode lasers for four-color, six-parameter detection. Acquisitions and analyses were performed using Cell Quest software (BDIS). Fluorescence voltages and compensation values were determined using single fluorochrome-stained cells from a healthy volunteer. After the acquisition of events in a live lymphocyte gate using forward and side scatter properties, CD3+/CD8+ lymphocytes were gated and represented in a CD28 and CD57 dot-plot (Figure 1A). Lymphocyte subpopulations were analyzed by the percentage of CD8+ T lymphocytes expressing different combinations of CD57 and CD28.
(A) Graphic representation of CD8+ T lymphocytes (using CD3+CD8+ dual staining over a lymphocyte gate) from CMV seronegative and CMV seropositive healthy subjects. Expression of CD28 and CD57 were essentially mutually exclusive. (B) Distribution of CD28 and CD57 in 9 CMV seronegative (CMV−) and 24 CMV seropositive (CMV+) healthy subjects. Statistically significant differences were observed in the distribution of both subsets correlating with CMV serostatus (arrows) □ Median; □ 25%–75%; ⊤ Non-Outlier Min-Max; ○ Outliers; ✚ Extremes
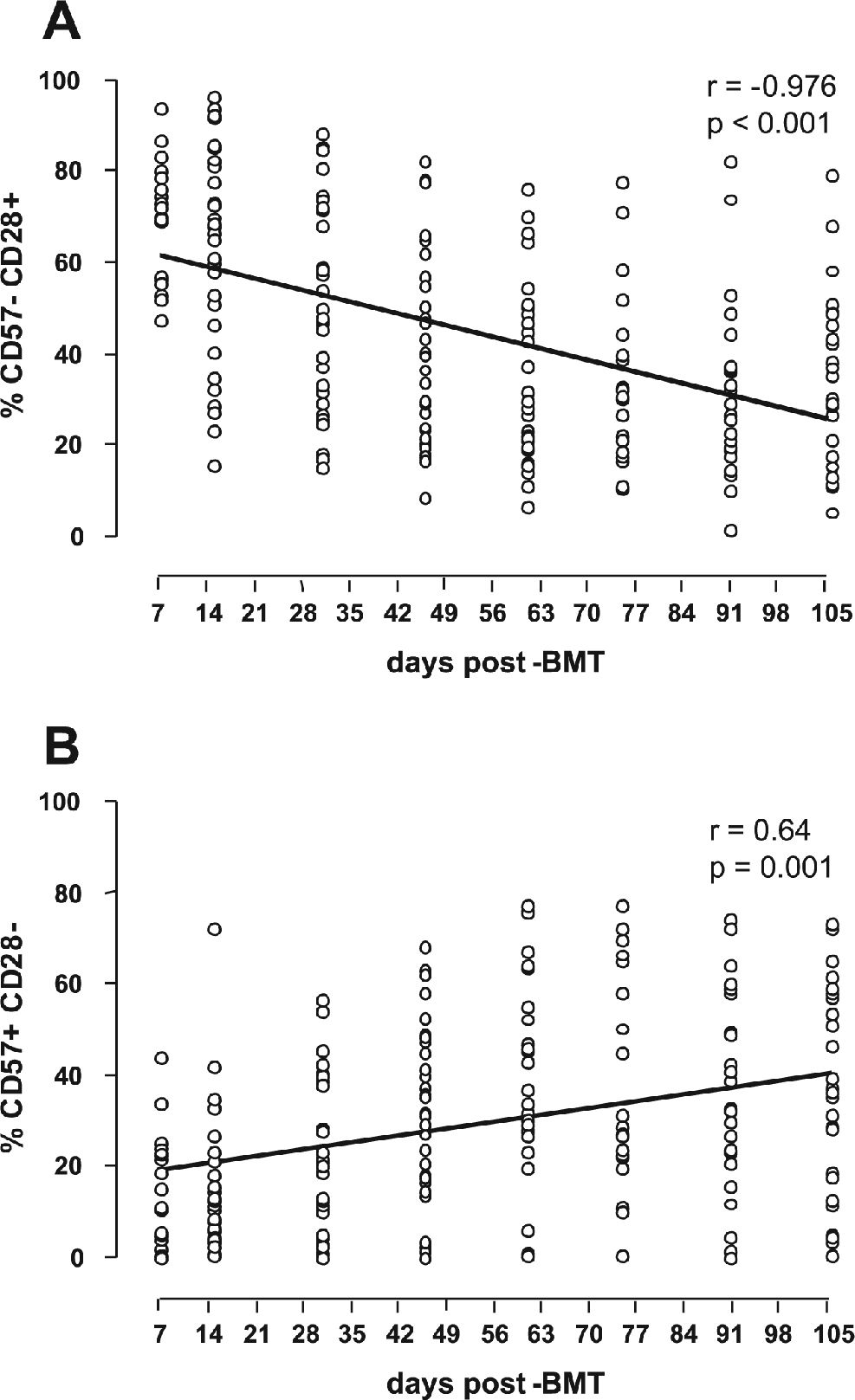
Results were plotted and analyzed using Microsoft Excel 2000 (Microsoft Corporation, Seattle, WA, USA), Statistic (StatSoft, Tulsa, OK, USA) and S-Plus (Mathsoft, Inc., Seattle, WA, USA). Group comparisons of pre-BMT, post-BMT, and final follow-up time points were performed using non-parametric Mann-Whitney tests, and p < 0.05 was considered to be statistically significant. Changes in the percentages of each lymphocyte subpopulation among CD8+ T cells occurring during the 120-day follow-up period after BMT were summarized using linear regression models, and patients within each specific subgroup were compared using Student’s t-tests applied to the regression slopes. These summaries were then used to compare subgroups. Spearman tests were used to correlate time post-BMT with relative changes in CD28+CD57− and CD28−CD57+ subsets.
RESULTSBMT patients datasetFrom April 1998 to March 2000, 45 patients underwent BMT, and 33 were enrolled in this study. Five patients did not sign the informed consent form. Four patients died within the first 35 days after BMT and were excluded, and three patients were lost to follow-up. Therefore, 33 patients were included in the analysis. Patient characteristics are shown in Table 1.
Active CMV infection and diseaseTwenty-three out of 33 BMT recipients (69.7%) had at least one episode of positive antigenemia (≥ 1 positive cell). Six of the 11 patients in the autologous BMT group (54.5%) and 17 of the 22 allogeneic BMT recipients (77.3%) developed positive antigenemia within 120 days post-BMT. The median time of antigenemia occurrence was 42.5 days in the autologous BMT recipients and 56 days in the allogeneic BMT recipients. The number of positive cells detected by antigenemia assays was higher in the allogeneic BMT recipients (median four positive cells, ranging from one to 1064) than in the autologous BMT recipients (median one positive cell, ranging from one to two cells; p < 0.01, Mann-Whitney test).
Of the 23 patients with positive antigenemia, two developed CMV disease during the 120-day follow-up period (8.7%). Both were allogeneic BMT recipients. One patient developed CMV pneumonia, and the other developed fever and pancytopenia. Interestingly, the first patient demonstrated a sharp drop in the percentage of CD57+CD28−CD8+ T lymphocytes at the onset of antigenemia. However, this lymphocyte population could not be analyzed in the second patient due to the low number of lymphocytes present during the pancytopenia.
Temporal occurrence of GVHD and CMV viremiaAcute GVHD, grade II or higher, was diagnosed in 12 allogeneic BMT recipients (54.5%). In the 17 allogeneic BMT recipients with positive antigenemia, GVHD was diagnosed in ten patients (58.8%). In the five allogeneic BMT recipients with negative antigenemia, GVHD was present in two patients (40%). The median time to diagnosis of GVHD was 26 days and this preceded the occurrence of CMV antigenemia, which took place at a median of 56 days. The occurrences of GVHD as well as CMV viremia and disease are described in Table 2.
Patient clinical descriptions
| Clinical description | Group | Number of patients (%) | Total |
|---|---|---|---|
| Follow-up time | 60–120 days | 7 (21.2%) | 33 |
| ≥120 days | 26(78.8%) | ||
| CMV antigenemia | Positive* | 23 (69.7%) | 33 |
| Negative** | 10 (30.3%) | ||
| CMV disease | Yes | 2 (6.1%) | 33 |
| No | 31 (93.9%) | ||
| Acute GVHD | Grade 0 or I | 10 (45.5%) | 22 |
| Grade II to IV | 12 (54.5%) | ||
| Outcome | Alive at day 120 | 32 (97.0%) | 33 |
| Death due to CMV disease | none | ||
| Death due to other causes at day 120 | 1 (3.0%) |
CMV: cytomegalovirus; GVHD: Graft-versus-host disease.
To evaluate the expression of CD28 and CD57 markers in CD8+ T lymphocytes, flow cytometric analyses were performed on EDTA-treated peripheral blood. The expression of CD28 was essentially mutually exclusive with CD57 in CD8+ T cells, such that a dual positive population was very rare (Figure 1A). To evaluate whether the expansion of the CD57+ cells was related to CMV, and to determine if the presence of CMV-specific antibodies was correlated with the distribution of CD8+ T lymphocyte subsets, 33 healthy volunteers were analyzed. As shown in Figure 1B, CMV-seropositive subjects presented with a higher percentage of CD57+ cells and a lower percentage of CD28+ cells, compared to CMV-seronegative subjects.
After BMT, CD28+CD8+ T lymphocytes continuously decrease, while CD57+CD8+ T lymphocytes continuously increaseUsing a linear regression model, the dynamics of the CD28+ and CD57+ subpopulations within CD8+ T lymphocytes were analyzed. In general, after BMT, the percentage of CD28+ cells decreased, while that of CD57+ cells increased. These changes occurred at a relatively constant rate, independent of transplant type or the occurrence of CMV viremia or GVHD (Figure 2). On average, the baseline level of CD28+ cells (measured early during engraftment) was higher in the allogeneic BMT patients (72.11% at baseline, with −0.38%/day thereafter) than in the autologous BMT patients (46.23% at baseline, with −0.17%/day thereafter). Consistent with their mutually exclusive expression, the baseline level of CD57+ was lower in the allogeneic BMT group (14.40% at baseline, with +0.27%/day thereafter) than in the autologous BMT group (18.42% at baseline, with +0.12%/day thereafter).
Influence of GVHD on CD57+CD8+ T lymphocytesThe presence of GVHD was also analyzed as a possible event influencing the changes seen in the CD8+ T lymphocyte subpopulations. Although both GVHD and CMV antigenemia were frequently diagnosed in the present series, they occurred at distinct times (day 26 vs. day 56), allowing us to examine independently the impact of each factor on changes in the T lymphocyte subpopulations. The occurrence of GVHD in 12 allogeneic patients had an impact on the CD57+ subset, triggering an increase of 4.9% in CD57+ lymphocytes (p < 0.05) (Table 3). However, daily changes in both the CD28+ and CD57+ populations were also observed in patients without GVHD, possibly reflecting immunologic reconstitution, as observed in patients without CMV viremia (Table 3).
Distribution of CD8+ T lymphocyte subpopulations at baseline and after allogeneic BMT, calculated daily changes during follow-up, and influence of GVHD
| Group (number of patients) and lymphocyte subset | Baseline (% of CD8+ T lymphocytes) | Calculated daily change (% of CD8+ T lymphocytes) | Change with GVHD (% of CD8+ T lymphocytes) |
|---|---|---|---|
| Allogeneic recipients without GVHD (n=10) | |||
| CD28+ | 68.46 | − 0.33* | - |
| CD57+ | 12.99 | + 0.30* | - |
| Allogeneic recipients with GVHD (n=12) ** | |||
| CD28+ | 68.00 | − 0.34* | − 3.23 |
| CD57+ | 19.22 | + 0.22* | + 4.90* |
| Allogeneic recipients who developed a positive CMV antigenemia (n=10) | |||
| CD28+ | 65.80 | − 0.33* | − 2.04 |
| CD57+ | 20.10 | + 0.23* | + 4.13* |
CMV: cytomegalovirus; GVHD: graft versus host disease.
The subset of patients who developed GVHD was analyzed for the impact of positive CMV antigenemia on the distribution of CD57+CD28−CD8+ T lymphocytes. The majority of patients who developed GVHD had at least one positive CMV antigenemia assay during the follow-up period (10 out of 12, or 83.3%) (Table 4).
Distribution of CD8+ T lymphocyte subpopulations at baseline and after BMT, calculated daily changes during follow-up, and influence of CMV antigenemia, with patients stratified by type of BMT
| Group (number of patients) and lymphocyte subset | Baseline (% of CD8+ T lymphocytes) | Calculated daily change (% of CD8+ T lymphocytes) | Change at onset of antigenemia (% of CD8+ T lymphocytes) |
|---|---|---|---|
| Without detectable CMV antigenemia (n=10) | |||
| CD28+ | 63.29 | − 0.18* | - |
| CD57+ | 8.66 | + 0.12* | - |
| With detectable CMV antigenemia (n=23) | |||
| CD28+ | 60.40 | − 0.30* | − 5.95* |
| CD57+ | 20.20 | + 0.20* | + 5.60* |
| Autologous (n=6) | |||
| CD28+ | 44.90 | − 0.16* | − 2.18 |
| CD57+ | 23.31 | + 0.11 | + 2.75 |
| Allogeneic (n=17) | |||
| CD28+ | 66.71 | − 0.34* | − 7.60* |
| CD57+ | 18.20 | + 0.25* | + 5.93* |
| Allogeneic recipients who developed GVHD (n=12) | |||
| CD28+ | 65.80 | − 0.33* | − 7.30* |
| CD57+ | 20.10 | + 0.23* | + 5.34* |
| Allogeneic recipients without GVHD but with detectable CMV antigenemia (n=7) | |||
| CD28+ | 68.46 | − 0.33* | − 5.32* |
| CD57+ | 12.99 | + 0.30* | + 5.47* |
CMV: cytomegalovirus; GVHD: graft-versus-host disease.
To evaluate whether the occurrence of CMV viremia exerted an effect on the distribution of CD28+ and CD57+ lymphocytes, the patients who experienced positive antigenemia during the follow-up period were compared to those who did not have any CMV-related episodes after BMT. The occurrence of positive antigenemia was associated with a steep drop in the percentage of CD28+ lymphocytes (5.95%, p < 0.01), along with an increase of CD57+ lymphocytes (5.60%, p < 0.01) (Table 4). Representative cases depicting the association of CMV antigenemia with CD8+ T cell subset changes are shown in Figure 3.
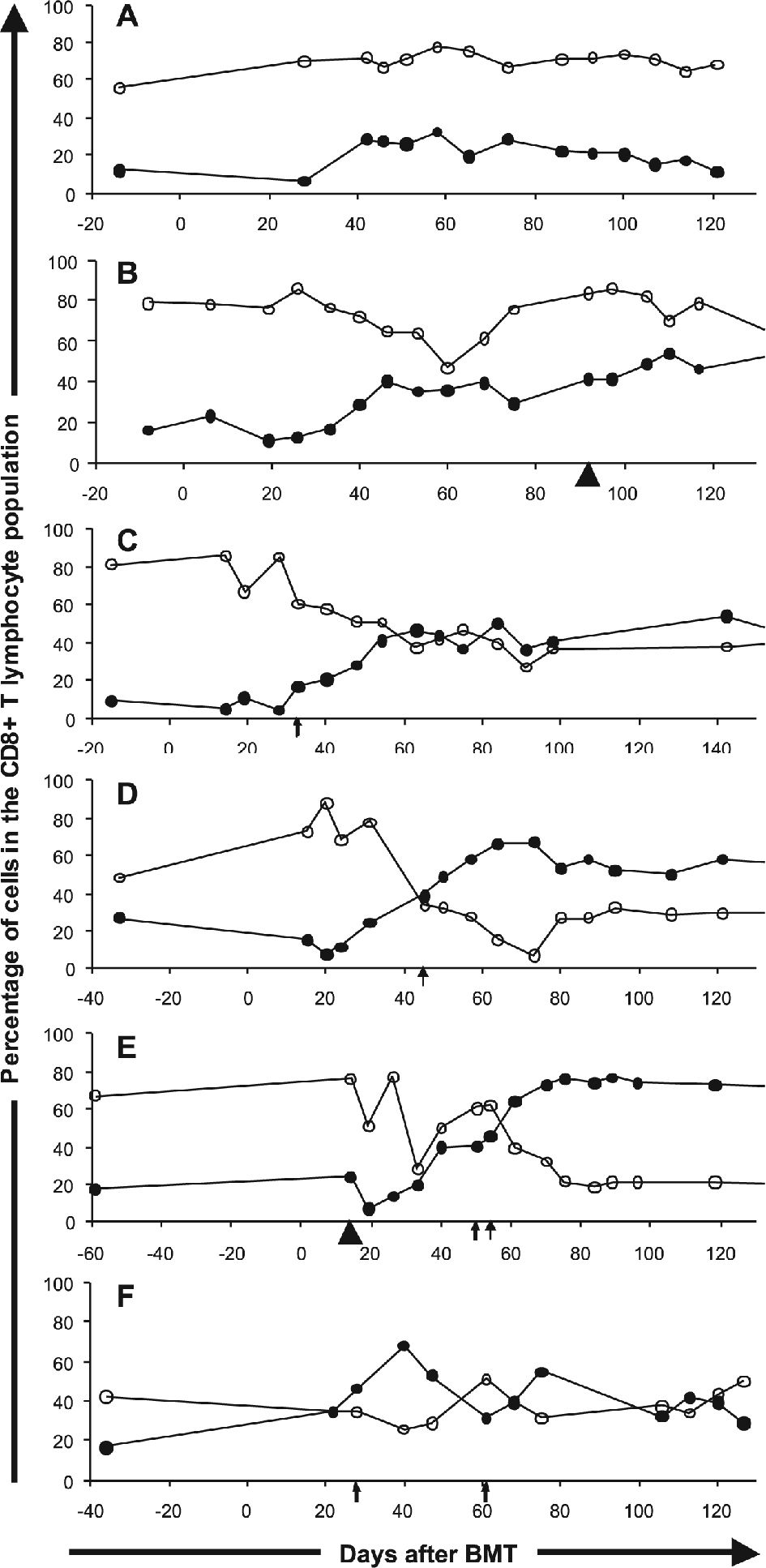
CD28+ (open circles) and CD57+ (closed circles) subpopulation percentages among CD8+ T lymphocytes in selected BMT recipients. Arrows represent positive antigenemia and arrowheads represent GVHD. (A) Allogeneic BMT in a patient who did not develop CMV antigenemia or GVHD. (B) Allogeneic BMT in a patient who developed GVHD. (C) and (D) Allogeneic BMT in two patients with CMV antigenemia. (E) Allogeneic BMT in a patient who developed both GVHD and CMV antigenemia at distinct moments during follow-up. (F) Autologous BMT in a patient developing CMV antigenemia on two occasions during follow-up
The abrupt changes observed in these subpopulations did not influence the calculated daily rate of change for CD28 or CD57 expression observed before the CMV viremia. Thus, after the CMV event, the daily rates of change of both CD28 and CD57 expression returned to the same pattern present prior to the viremia. This strongly suggested that the abrupt changes were related to the antigenemia rather than to a natural phenomenon associated with immune reconstitution after transplant.
The CMV-triggered changes in CD8+ T lymphocyte subsets occurred primarily in the allogeneic BMT patients, as shown in Table 3. In addition, in the seven patients without GVHD who experienced positive antigenemia, a significant increase in the CD57+ subpopulation could also be observed (Table 4). Two examples are shown in figures 3B and 3E. Figure 3B represents a patient with GVHD and no detectable CMV antigenemia who experienced an increase in the CD57+ subset, while Figure 3E represents a patient who developed both GVHD, which had minor effects on the CD57+ subset, and CMV antigenemia, which had a more pronounced effect on both the CD57+ and CD28+ subsets.
DISCUSSIONIn this prospective study, we confirmed a correlation between CMV seropositivity and relative expansion of the CD57+CD28−CD8+ T lymphocyte population in healthy volunteers. We also took advantage of the high incidence of CMV viremia in BMT patients to study the temporal association between CMV viremia and the relative expansion of this cell subset. In addition to describing the close relationship between CMV reactivation and expansion of this CD8+ subset in BMT recipients,31,32 we were also able to detect an effect of GVHD on the CD57+CD28−CD8+ T lymphocyte subpopulation.
CD57, formerly known as leu7, is a glycoprotein found mostly on NK cells and CD8+ T lymphocytes. It is thought to identify a late memory effector subset of CD8+ T lymphocytes.14,33,34 The increase in this subset after solid organ transplantation35 or BMT36 has been described previously, and these cells have been shown to be of donor origin, as the myeloablative conditioning regimens used prior to transplant completely eliminate recipient T-cells.37 We were able to confirm this result and also to demonstrate that this increase happens in a slow and continuous fashion, continuing up to four months after BMT, independently of CMV viremia or GVHD diagnosis. Together with the increase in the CD57+CD28− subset, a slow and continuous decrease in the CD57−CD28+ subset was also observed. This behavior possibly reflects an immunologic reconstitution process that occurs after severe induced immunodeficiency. During the first 120 days following BMT, that is characterized by pronounced immune system suppression, patients face repeated immunological stimuli through re-exposure to and/or reactivation of infectious agents and, with allogeneic BMTs, exposure to alloantigens. Once activated to begin the maturation process, CD8+ T lymphocytes in BMT recipients tend to lose CD28 expression and, as we have demonstrated, acquire the expression of the CD57 surface molecule.
More recently, investigators have described an increased production of interferon gamma (IFNγ), either in response to mitogens38 or CMV-derived antigens,34 and an oligoclonal, CMV-specific expansion39,40 in the CD57+CD8+ T lymphocyte subset. Furthermore, Khan et al. have demonstrated that CMV-specific CD8+ cells with cytotoxic activity more frequently express surface CD57 than they do CD28.41 Thus, while some have suggested that these cells have undergone replicative senescence or clonal exhaustion and lack a protective function,42 other authors have proposed that this subset plays a regulatory role, modulating immune responses to large, harmful antigenic stimuli.43 Still others have suggested that these cells have a cytotoxic, protective role, characterized by production of IFN-γ, IL-2, and IL-4 and by expression of high levels of perforin.41,42,44
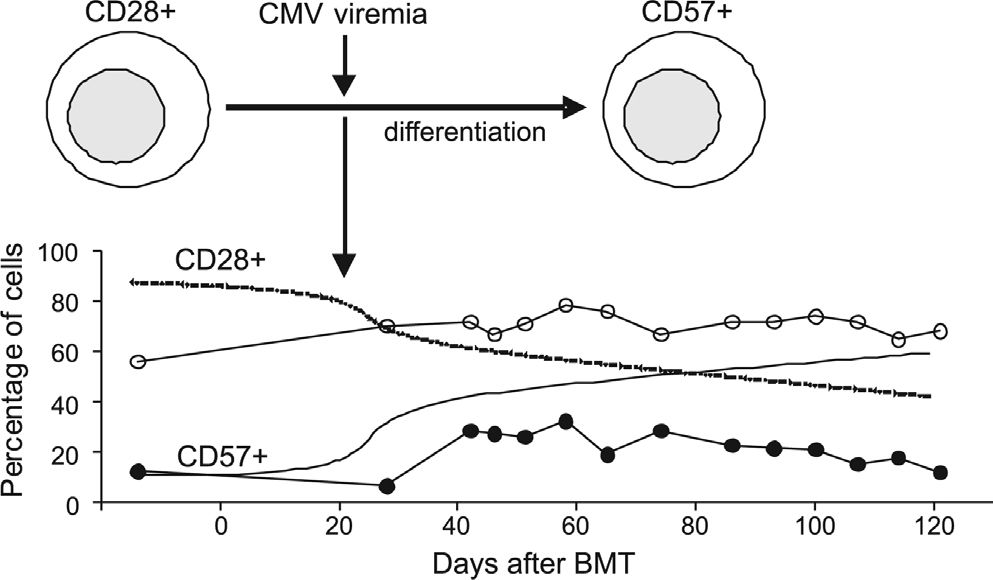
An association between expansion of the CD57+CD8+ T lymphocyte subset and CMV infection in BMT was first suggested by Dolstra et al., who described a higher number of these cells in those patients that developed CMV infection.45 In two patients, CMV-specific cytotoxicity activity was also demonstrated in vitro46. Our findings strongly suggest an association between CMV viremia and the relative expansion of this subset, with an impressive increase of greater than 6% at the time of CMV antigenemia occurrence. The observed temporal association between CMV viremia and the relative increase in the CD57+CD28−CD8+ T lymphocyte subset suggests the presence of an antigen-specific response driven by CMV (Figure 4). However, when patients were divided into allogeneic and autologous BMT groups, we could demonstrate this association only in the allogeneic BMT group.
Proposed model for CD8+ T lymphocyte subset reconstitution after BMT and the effect of CMV viremia. A continuous relative decrease in CD28+ cells, along with a continuous relative increase in CD57+, takes place after BMT engraftment. The occurrence of CMV viremia exerts a substantial effect on this system, causing a steep incline in both curves
Autologous BMT recipients had a lower percentage of CD28+ cells (46.23% vs. 72.11%) and a higher percentage of CD57+ cells (18.42% vs. 14.4%) shortly after transplantation, possibly reflecting a better ability to contain CMV viremia, which, again, was consistently less prevalent here than in the allogeneic BMT group. The early availability of this antigen-specific T cell subset may account for the lower incidence of CMV viremia and the lower levels of antigen detection in the case of CMV reactivation, that would result in smaller relative changes in both the CD28+ and CD57+ subsets. We were not able, however, to correlate an increased proportion of CD57+ cells with a lower incidence of CMV reactivation in the allogeneic BMT recipients. Analysis of these cases revealed that some patients with high levels of CD57+ T cells did reactivate. Those patients that did so were generally those who developed acute GVHD (higher than grade II) (Figure 3E).
Thus, it appeared that the expansion of CD57+CD28−CD8+ T lymphocytes was not exclusively related to CMV viremia. In fact, GVHD had a similar effect on the relative increase of this cell population. Acute GVHD is a frequent and early event in the course of allogeneic BMT, usually preceding antigenemia by several weeks. In our cohort, acute GVHD occurred at day 26 post-BMT, while antigenemia was diagnosed at day 56 post-BMT. This difference allowed us to independently analyze the effects of these two phenomena. GVHD involves donor-derived cytotoxic CD8+ T lymphocytes reacting against recipient tissues, and results in dramatic immune cell activation.47 The striking association between GVHD and increased CD57 expression in the present study suggests an additional pathway for CD8+ T lymphocyte differentiation, probably triggered by alloantigens. Other groups have previously described an increase in the CD28− subset of CD8+ lymphocytes related to GVHD, but none evaluated expression of the CD57 marker.48,49 A more recent study observed an increase in CD57+CD8+ T cells in pediatric BMT recipients with a diagnosis of GVHD but was not able to demonstrate a temporal correlation between the increase of this subset and GVHD diagnosis.42 In contrast, we did not find a marked effect of GVHD on the CD28+ subset, as was previously suggested by Lee et al.48
We were able to demonstrate continuous relative changes in the CD28+ and CD57+ subsets during the first 120 days after both allogeneic and autologous BMTs, as part of immune reconstitution and maturation post-BMT. More importantly, a clear correlation was observed between the expansion of the CD57+CD28−CD8+ T lymphocyte subpopulation and CMV viremia, suggesting an important role for this cell subset in the context of CMV disease. Supporting this idea, the expansion of this cell population was associated with the appearance of CMV viremia and was generally followed by a retraction coincident with resolution of the viremia. However, further studies are needed to address whether CD57+CD8+ T cells have a protective role in the control of CMV infection and disease, or whether they are a hallmark of the chronic antigenic stimulation induced by CMV.21,26
We thank Marta Anesia Viana, Milena Brunialti, Helena Tomiyama, and Lucy Vilas Boas for their continuous support during the laboratory work, as well as the nursing staff of the Bone Marrow Transplant Program of The Hematology and Hemotherapy Discipline of the University of São Paulo for helping identify patients and collect specimens during this project.