Intravenous infusion of crystalloid solutions is a cornerstone of the treatment of hemorrhagic shock. However, crystalloid solutions can have variable metabolic acid-base effects, perpetuating or even aggravating shock-induced metabolic acidosis. The aim of this study was to compare, in a controlled volume–driven porcine model of hemorrhagic shock, the effects of three different crystalloid solutions on the hemodynamics and acid-base balance.
METHODS:Controlled hemorrhagic shock (40% of the total blood volume was removed) was induced in 18 animals, which were then treated with normal saline (0.9% NaCl), Lactated Ringer's Solution or Plasma-Lyte pH 7.4, in a blinded fashion (n = 6 for each group). Using a predefined protocol, the animals received three times the volume of blood removed.
RESULTS:The three different crystalloid infusions were equally capable of reversing the hemorrhage-induced low cardiac output and anuria. The Lactated Ringer's Solution and Plasma-Lyte pH 7.4 infusions resulted in an increased standard base excess and a decreased serum chloride level, whereas treatment with normal saline resulted in a decreased standard base excess and an increased serum chloride level. The Plasma-Lyte pH 7.4 infusions did not change the level of the unmeasured anions.
CONCLUSION:Although the three tested crystalloid solutions were equally able to attenuate the hemodynamic and tissue perfusion disturbances, only the normal saline induced hyperchloremia and metabolic acidosis.
Hemorrhagic shock is a well-known cause of metabolic acidosis, which is associated with a poor prognosis.1 Hypothermia, coagulopathy, and acidosis comprise what has been termed the “trauma triad of death.”2
Intravenous infusion of crystalloid solutions is a cornerstone of hemorrhagic shock treatment, and it can both restore the intravascular compartment and maintain cardiac output and tissue perfusion.3 However, crystalloid solutions can have variable metabolic acid-base effects, sometimes perpetuating or aggravating metabolic acidosis.4 Normal saline is a very frequently used solution,5 but its use has been associated with the development of hyperchloremic acidosis.6,7 The use of so-called “balanced solutions,” which are more similar to human plasma in terms of electrolytes and acid-base chemistry, may avoid this problem.4 Lactated Ringer's (LR) Solution and Plasma-Lyte pH 7.4 are two balanced solutions that are commonly used in clinical practice.
To our knowledge, no study has compared the effects of these three solutions on the quantitative acid-base and hemodynamic profiles in a hemorrhagic scenario. Therefore, the aim of this study was to compare, in a controlled, volume-driven porcine model of hemorrhagic shock, the effects of three different crystalloid solutions on the hemodynamics and quantitative acid-base balance.
MATERIALS AND METHODSAnimalsAll experiments were carried out in accordance with the National Institutes of Health (1985) and the American Physiological Society (1995) principles on the care, manipulation, and protection of laboratory animals, after the approval of the Animal Ethics Committee from Hospital Israelita Albert Einstein, São Paulo, Brazil.
Eighteen healthy, male white pigs (30.4±4.2 kg) were used in the experiments. The animals were evaluated by a veterinarian before and during all experiments. Access to food was suspended 12 hours prior to surgery, but free access to water was always provided.
Surgical proceduresAnimals were anesthetized using the following protocol: the pigs were pre-medicated with ketamine (10 mg/kg, i.m.) and midazolam (0.25 mg/kg, i.m.). Propofol (5 mg/kg i.v.) was used to induce anesthesia, which was maintained with a continuous i.v. infusion of fentanyl (2.5 μg/kg/h), pancuronium (0.4 mg/kg/h) and isoflurane (1.5%).
After induction, the animals were orotracheally intubated (6.5–7.0 tubes) and mechanically ventilated (Origami Takaoka, K. Takaoka, Brazil) using the volume assist-control mode with the following parameters: tidal volume, 10 ml/kg body weight; positive ending expiratory pressure (PEEP), 5 cmH2O; and 25–30% of fractional inspiratory O2 concentration (FiO2). The goal was to maintain a normal partial pressure of oxygen (PaO2, 60–100 mmHg) in an arterial blood sample. The respiratory rate (RR) was adjusted to obtain a normal carbon dioxide partial pressure (PaCO2, 35–45 mmHg) in an arterial blood sample. Thermal blankets were used to keep the core temperature around 38°C.
During all anesthetic and surgical procedures, continuous electrocardiography monitoring was performed (Dixtal 2021 monitor, Brazil). Arterial and venous lines were placed via left inguinal (femoral) dissection. The arterial line was connected to a pressure transducer (Edwards Lifesciences, USA) to provide continuous arterial pressure measurements (Dixtal 2021 monitor, Brazil). The right jugular vein was also dissected to allow for pulmonary artery catheterization (7F Swan-Ganz, Edwards Lifesciences, USA) and continuous cardiac output measurement (Vigilance, Edwards Lifesciences, USA).
A mini-laparotomy and a cystostomy were performed in all animals to quantify urinary output.
Experimental protocolDuring surgical preparation, 20 ml/kg of normal saline was administered to all animals. After performing the sedation, mechanical ventilation, and vascular access, controlled hemorrhagic shock was induced in the animals. Based on the calculated ratio of 76 ml blood/kg of body weight,8 we estimated the total blood volume to guide the exsanguination process. Forty percent of the total blood volume was removed via manual blood withdrawal through the arterial catheter in three equal aliquots, with a 30-minute interval between withdrawals. The animals were then randomly allocated to one of the three resuscitation groups (six animals per group): Normal Saline (0.9% NaCl, Baxter Viaflex, Brazil), LR solution (Lactated Ringer's Solution, Baxter, Viaflex, Brazil), or Plasma-Lyte pH 7.4 (Baxter Healthcare Corporation, USA) in a blinded fashion. The composition of the solutions is described in Table 1. We started the resuscitation with one of the three crystalloid solutions one hour after the bleeding was completed. The total volume infused was three times the total blood volume removed. The total time to infuse the crystalloid solution was 10–15 min per animal, and the infusion was performed using a pressurized system (300 mmHg).
Characteristics of the fluids used for resuscitation.
| Normal Saline | Lactated Ringer's Solution | Plasma-Lyte pH 7.4 | |
|---|---|---|---|
| Sodium (mEq/l) | 154 | 130 | 140 |
| Potassium (mEq/l) | 0 | 4 | 5 |
| Calcium (mEq/l) | 0 | 3 | 0 |
| Magnesium (mEq/l) | 0 | 0 | 3 |
| Chloride (mEq/l) | 154 | 109 | 98 |
| Lactate (mEq/l) | 0 | 28 | 0 |
| Gluconate (mEq/l) | 0 | 0 | 23 |
| Acetate (mEq/l) | 0 | 0 | 27 |
| Osmolarity (mOsm/l) | 308 | 275 | 294 |
| pH | 5.50 | 6.75 | 7.40 |
The heart rate, blood pressure, cardiac output and SvO2 were monitored during all phases of the experiment: baseline, after each blood withdrawal, 30 min after finishing the bleeding process, immediately after resuscitation and 1 hour after resuscitation. Blood samples were collected before the first blood withdrawal, 1 hour after the final blood withdrawal and 1 hour after the resuscitation process. After the protocol was complete, the animals were euthanized via anesthetic overdose followed by injection of a 20 ml KCl (19.1%) i.v. bolus.
Laboratory samplesArterial blood samples were collected in heparinized syringes and analyzed using a blood-gas analyzer (ABL 700, Radiometer, Copenhagen, Denmark). The analyzer measured samples at 37°C. We collected the following data from the analyzer output: pH, partial pressure of carbon dioxide, bicarbonate, standard base excess (SBE), and ionized calcium. The plasma sodium, chloride, lactate, and potassium levels were measured using ion-selective electrodes in the blood-gas analyzer. The machine calculated the bicarbonate concentration using the Henderson–Hasselbach equation. The SBE was calculated according to Wooten's equation,9 the formula least sensitive to PCO2, because our experiment was prone to large variations in the PCO2. Additionally, 5 ml of plasma was collected and stored at -80°C. The magnesium (calorimetric method using Formazan dye and Randox blue xylitol, Vitros System, Johnson & Johnson, USA), albumin (calorimetric method using Randox Bromocresol green, dry chemistry, Vitros System, Johnson & Johnson, USA), and phosphate (calorimetric method and dry chemistry, Vitros System, Johnson & Johnson, USA) levels were measured in these plasma samples.
Conceptual framework for acid-base analysisTo separate the independent components of the acid-base disturbances, we performed a quantitative physicochemical analysis using Stewart's methodology, as modified by Figge et al. and others.10–12 In this approach, the H+ concentration and, therefore pH, is determined using three independent variables: the strong ion difference (SID); the total concentration of weak acids (Atot), which is mainly determined by the albumin and phosphate levels; and the PCO2.
This method initially involves the calculation of the apparent strong ion difference (SIDa):
Because lactate is an independent determinant of mortality in critically ill patients following ICU admission,13,14 the SIDa was “partitioned” into the inorganic ion difference ([Na+] + [K +] + [Mg2+] + [Ca2+] - [Cl−]) and the plasma lactate level.
Alternatively, the SID can be calculated by taking into account the role of weak acids (e.g., CO2, albumin, and phosphate) in the balance of electrical charges in the plasma water. This method results in the effective strong ion difference (SIDe). The formula for SIDe, as determined by Figge et al.,12 is as follows:
PCO2 is measured in mmHg, albumin is measured in g/L, and phosphate is measured in mmol/L.
The difference between the SIDa and SIDe should equal 0 (electrical charge neutrality), unless there are unmeasured charges to explain this “ion gap.” Such charges are described by the strong ion gap (SIG):11
A positive SIG represents unmeasured anions (e.g., sulfate, keto acids, citrate, pyruvate, acetate, gluconate, etc.), which must be included to account for the measured pH.
Statistical analysisThe data were assessed for a normal distribution using the Kolmogorov-Smirnov goodness-of-fit model and are presented as the mean and standard deviation. The baseline mean values were compared using a one-way analysis of variance (one-way ANOVA), with a post-hoc Tukey's test. Hemodynamic, oxygen and laboratory variables were compared by two-way ANOVA, focusing on the group vs. time interaction. The significance level was set at p<0.05 for single comparisons. The commercially available SPSS 10.0 statistical package (Chicago, Illinois, USA) was used to perform the statistical analysis.
RESULTSTable 2 shows the baseline characteristics for the animals in each experimental group. The groups were not different with respect to any of the studied characteristics, except for a slightly higher SIG in the Plasma-Lyte pH 7.4 group when compared to the LR group.
Baseline metabolic characteristics of the groups.
| Characteristics | Normal Saline (n = 6) | Lactated Ringer's Solution (n = 6) | Plasma-Lyte pH 7.4 (n = 6) | p-value ∗ |
|---|---|---|---|---|
| pH | 7.39±0.06 | 7.40±0.06 | 7.43±0.05 | 0.593 |
| PaCO2 (mmHg) | 44±7 | 42±2 | 39±5 | 0.253 |
| Bicarbonate (mEq/l) | 26.4±2.7 | 25.8±2.0 | 25.0±1.5 | 0.564 |
| SBE (mEq/l) | 2.8±2.1 | 1.0±2.1 | 1.2±1.3 | 0.292 |
| SIDai (mEq/l) | 42.3±2.0 | 39.5±3.3 | 41.5±0.8 | 0.134 |
| Sodium (mEq/l) | 136.1±2.4 | 135.9±2.6 | 137.0±2.8 | 0.744 |
| Chloride (mEq/l) | 102±4 | 103±5 | 103±3 | 0.653 |
| Lactate (mEq/l) | 1.7±1.3 | 1.5±0.7 | 1.2±0.5 | 0.684 |
| Albumin (g/dl) | 2.4±0.3 | 2.2±0.4 | 2.4±0.2 | 0.581 |
| Hemoglobin (g/dl) | 10.6±0.8 | 9.7±0.9 | 11,0±1.0 | 0.068 |
| SIG (mEq/l) | 2.3±1.9 | 0.8±1.5 | 3.6±1.3 | 0.030 |
Data are expressed as the mean ± SD.
The mean blood volume removed in each group was 955 (±163) ml in the Normal Saline group, 925 (±127) ml in the LR group and 896 (±105) ml in the Plasma-Lyte pH 7.4 group, with no statistical difference among the three groups (p = 0.75).
The hemorrhage induced an increase in the heart rate, a decrease in the cardiac output and a trend towards a decrease in the blood pressure; these changes were of the same magnitude in the three groups. The resuscitation phase took less than 15 min for all animals. The total volume of fluid infused per animal was 2865 (±488) ml in the Normal Saline group, 2774 (±381) ml in the LR group and 2681 (±314) ml in the Plasma-Lyte pH 7.4 group, with no significant difference among the three groups with a single p-value reported. The three crystalloid solutions were equally able to attenuate the hemodynamic alterations (Table 3). The post-hoc analysis comparing the equilibrium and baseline time points showed a complete reversion of the decreased cardiac output (p = 0.25) and a partial reversion of the increased heart rate (p = 0.02). From the beginning of the hemorrhage until the beginning of the fluid resuscitation, all animals were anuric. The urine output after the beginning of fluid resuscitation was not different among the three groups: 218 (±147) ml/h for the normal saline group, 154 (±75) ml/h for the LR group, and 205 (±240) ml/h for the Plasma-Lyte pH 7.4 group (p = 0.753). Oxygen delivery (DO2) and consumption (VO2) and mixed oxygen saturation (SvO2) had similar patterns among the three groups. The decrease in the DO2 and SvO2 were only partially reversed following fluid infusion in all groups (equilibrium vs. baseline; p<0.01 for both variables).
Effects of crystalloid infusion on the hemodynamic and oxygenation parameters.
| ††Parameter | Group | Baseline | Hemorrhage | Equilibrium† | p-value ∗ |
|---|---|---|---|---|---|
| MAP | Normal SalineLactated Ringer's SolutionPlasma-Lyte pH 7.4 | 99.5±31.0107.5±24.2101.8±26.6 | 78.8±27.987.7±18.168.0±34.0 | 80.7±29.0107.5±31.885.7±33.2 | 0.125∗0.124# |
| HR | Normal SalineLactated Ringer's SolutionPlasma-Lyte pH 7.4 | 114.4±29.7143.0±28.8141.7±27.1 | 207.4±31.6208.8±25.1204.5±16.4 | 159.4±29.4168.2±40.8181.3±15.6 | <0.0001∗0.075# |
| CO | Normal SalineLactated Ringer's SolutionPlasma-Lyte pH 7.4 | 5.6±2.35.2±1.45.7±2.0 | 2.7±0.73.2±0.62.5±0.4 | 4.7±1.75.1±0.73.8±0.7 | <0.0001∗0.221# |
| PWP | Normal SalineLactated Ringer's SolutionPlasma-Lyte pH 7.4 | 10.0±3.512.8±6.69.6±3.0 | 6.7±2.59.5±3.77.8±2.2 | 7.3±2.510.4±4.38.8±1.7 | 0.620∗0.092# |
| SvO2 | Normal SalineLactated Ringer's SolutionPlasma-Lyte pH 7.4 | 70.5±7.270.3±5.574.5±6.3 | 37.9±6.246.3±3.444.9±12.6 | 53.8±7.546.2±17.057.1±11.7 | <0.0001∗0.283# |
| DO2 | Normal SalineLactated Ringer's SolutionPlasma-Lyte pH 7.4 | 541.6±164.3470.2±100.8604.6±233.9 | 253.6±39.2283.1±34.3244.9±67.0 | 360.8±136.4328.6±55.4321.8±73.9 | <0.0001∗0.762# |
| VO2 | Normal SalineLactated Ringer's Solution Plasma-Lyte pH 7.4 | 142.5 + 82.4119.3 + 12.3120.9 + 24.9 | 142.7 + 26.0136.3 + 19.4129.7 + 47.8 | 145.9 + 41.2140.8 + 23.9163.4 + 97.9 | 0.471∗0.827# |
Data are expressed as the mean ± SD.
# Two-way ANOVA for the factor Group (no statistical significance was found for the Time × Group interaction).
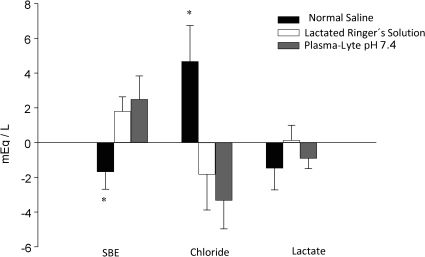
The three groups were similar with respect to all laboratory values recorded after hemorrhage, including SIG. The infusion of normal saline induced a negative SBE and a highly significant increase in the plasma chloride concentration, whereas the infusion of Ringer's lactate and Plasma-Lyte pH 7.4 induced a positive SBE and a negative plasma chloride concentration (Figure 1). The Plasma-Lyte pH 7.4 infusion did not induce the accumulation of unmeasured anions, as demonstrated by the SIG values (Table 4).
Effects of crystalloid infusion on the acid-base parameters.
| Parameter | Group+ | Hemorrhage | Equilibrium† | p-value∗ |
|---|---|---|---|---|
| pH | Normal SalineLactated Ringer's SolutionPlasma-Lyte pH 7.4 | 7.31±0.077.32±0.037.36±0.10 | 7.31±0.097.31±0.137.43±0.08 | 0.506 |
| PaCO2 (mmHg) | Normal SalineLactated Ringer's SolutionPlasma-Lyte pH 7.4 | 55±1250±344±10 | 51±1457±1840±8 | 0.425 |
| Bicarbonate (mEq/L) | Normal SalineLactated Ringer's SolutionPlasma-Lyte pH 7.4 | 26.8±3.225.0±2.023.7±1.1 | 24.6±3.026.8±2.326.0±1.1 | 0.045 |
| SBE (mEq/L) | Normal SalineLactated Ringer's SolutionPlasma-Lyte pH 7.4 | 2.0±3.10.3±2.0-0.8±1.2 | 0.96±2.972.11±2.571.67±1.05 | 0.042 |
| SIDai (mEq/L) | Normal SalineLactated Ringer's SolutionPlasma-Lyte pH 7.4 | 41.9±3.440.7±3.342.8±2.2 | 38.6±3.341.7±2.243.6±2.6 | 0.152 |
| Sodium (mEq/L) | Normal SalineLactated Ringer's SolutionPlasma-Lyte pH 7.4 | 135.5±1.8135.5±2.7136.1±3.4 | 136.8±1.9134.4±3.5134.7±3.5 | 0.388 |
| Chloride (mEq/L) | Normal SalineLactated Ringer's SolutionPlasma-Lyte pH 7.4 | 102±3103±5102±2 | 107±3101± 398±2 | 0.018 |
| Lactate (mEq/L) | Normal SalineLactated Ringer's SolutionPlasma-Lyte pH 7.4 | 3.0±1.32.5±1.22.8±0.6 | 1.5±0.52.7±1.81.9±0.4 | 0.213 |
| SIG (mEq/L) | Normal SalineLactated Ringer's SolutionPlasma-Lyte pH 7.4 | -0.1±2.71.0±1.74.7±1.9 | 2.7±1.82.0±2.47.4±2.3 | 0.511 |
Data are expressed as the mean ± SD.
In this study, we demonstrate, using an experimental model of hemorrhagic shock, that Plasma-Lyte pH 7.4 and LR Solution cause an increase in the SBE, while normal saline lowers the SBE. Applying a quantitative acid-base approach, we demonstrated that Plasma-Lyte pH 7.4 did not cause an accumulation of unmeasured anions. Accordingly, the difference in the SBE was mainly attributable to fluid-induced variations in the serum chloride level. The three solutions were equally effective in attenuating the macro-hemodynamic alterations. While the lactate levels tended to decrease following the administration of normal saline and Plasma-Lyte pH 7.4, but not LR solution, this difference was not statistically significant.
Hyperchloremic acidosis is a well-known phenomenon.15 Furthermore, in clinical practice, the intravenous infusion of chloride-rich solutions, such as normal saline, is commonly associated with the development of hyperchloremic acidosis.16 This association has been described in several scenarios, including in normal volunteers,6 but seems to be increased in patients with inflammation.17 After development, hyperchloremic acidosis takes a few days to resolve.18 However, the consequences of hyperchloremic acidosis are not well established.19 Some studies report no impact on clinical outcome,14 while other studies found an impact.10 Small studies have suggested that large infusions of normal saline may be detrimental to renal function.21 Despite the controversy regarding its impact on clinical outcome, hyperchloremia can have deleterious effects, such as increase in the tumor necrosis factor (TNF), interleukin (IL)-6, and IL-10 levels,22 a decrease in urine output,23 and coagulation disturbances.24,25 However, there is no definitive conclusion as to whether these deleterious effects are directly related to the hyperchloremia or if they are mediated by metabolic acidosis and/or acidemia.
The use of balanced solutions has been studied in some experimental and clinical situations. In experimental sepsis, Kellum et al.26 demonstrated that animals resuscitated with Hextend (a colloid based balanced solution) survived 45% longer and had significantly less metabolic acidosis than animals treated with normal saline. Furthermore, the survival time was positively correlated with the changes in pH and negatively correlated with the increase in serum chloride after initial resuscitation. Similar results were found in a massive hemorrhagic model. Rats resuscitated with normal saline were significantly more acidotic than rats resuscitated with an equal volume of LR and had significantly worse survival (50% vs. 100%, respectively).27 In a clinical study of elderly surgical patients,28 the use of LR solution and Hextend prevented the development of hyperchloremic metabolic acidosis and resulted in improved gastric mucosal perfusion when compared with saline-based solutions.
Few studies have specifically examined the use of Plasma-Lyte pH 7.4. Results similar to ours have been reported following its use in patients who underwent general surgery or kidney transplantation.21 It should be noted, however, that a quantitative approach was not used in these studies; therefore, the unmeasured anions could not be precisely estimated. Based on our results, Plasma-Lyte pH 7.4 and LR solution have the same general acid-base effect (SBE). This result is consistent with previous studies in renal transplantation patients, except when larger volumes were infused, such as in the pump prime during cardiopulmonary bypass.18 Importantly, Plasma-Lyte pH 7.4 did not cause an accumulation of unmeasured anions as evidenced by the sustained SIG values. Some concerns exist regarding the use of LR, especially due to its low osmolarity (255–273 mOsm/L). Some authors suggest that its use should be limited and should be avoided for cases at risk of intracranial hypertension.29 In addition, the induction or maintenance of hyperlactemia by LR solution (as suggested by our results) may be significant when larger volumes are infused. Our study suggests that Plasma-Lyte pH 7.4 is a safe alternative for the treatment of hemorrhagic shock.
Perhaps the most important point of our study is the use of the metabolic acidosis magnitude as a clinical monitoring tool. According to our results, resuscitation with normal saline can disrupt the relationship between base deficit and hypovolemia (an increased base deficit during resuscitation). The misdiagnosis of worsening acidosis as tissue hypoperfusion can be a factor leading to the overhydration of many patients after major surgical procedures and is correlated with the length of the intensive care unit stay.16 Fluid overload has also been associated with a prolonged time on mechanical ventilation30 and a lower survival rate31 in critically ill patients. Avoidance of normal saline, whenever base deficit needs to be monitored, can prevent some of these problems. A similar consideration can be made with respect to LR solution and the serum lactate levels; however, based on our results and the results of others, this point seems to be less important.
Our study has some limitations. First, our model caused only low-level metabolic acidosis in the majority of the animals. We would probably need to prolong the hypotensive period to achieve a more significant acidosis, but this extension could lead to death before resuscitation. Because the shock-induced acidosis was not fundamental to our study, we do not believe its absence is a significant limitation to the interpretation of the results. Second, we could not a priori define the sample size needed to detect potential differences because there have been no similar studies that we could use to estimate the magnitudes of the effects. Therefore, we cannot exclude the possibility that Plasma-Lyte pH 7.4 causes a greater increase in the SBE than LR solution. However, if this difference does exist, it is probably of a clinically irrelevant magnitude.
In summary, the three crystalloid solutions were able to reverse the hemorrhagic shock–induced hemodynamic disturbances. While normal saline was associated with hyperchloremic acidosis, LR and Plasma-Lyte pH 7.4 did not result in hyperchloremic acidosis and led to a more acceptable metabolic profile. Based on our results, Plasma-Lyte pH 7.4 should be considered a reasonable alternative in the treatment of hemorrhagic shock. However, future clinical studies should address this specific point.
This is the first study that simultaneously compared the effects of three different crystalloid solutions on acid-base and hemodynamic profiles following hemorrhagic shock. Although the three crystalloid solutions tested were equally able to attenuate the hemodynamic and tissue perfusion changes following induction of hemorrhagic shock, only normal saline induced hyperchloremia and metabolic acidosis.
No potential conflict of interest was reported.
Noritomi DT, Pereira AJ, and Silva E conceived and designed the study, conducted the experiment, performed the analysis of the results, reviewed the drafts of the manuscript, and approved the final version of the manuscript. Bugano DDG and Rehder PS conducted the experiment, reviewed the drafts of the manuscript, and approved the final version of the manuscript.